Close the Gaps
Controlling critical data and documenting processes can quickly get lost in the weeds at high-growth MedTech companies. What no one tells you is how gaps in these activities impact your commercialization journey. Add in regulatory and compliance requirements to your development goals, and it only gets more challenging. Just a QMS isn’t enough anymore. Your teams need more.
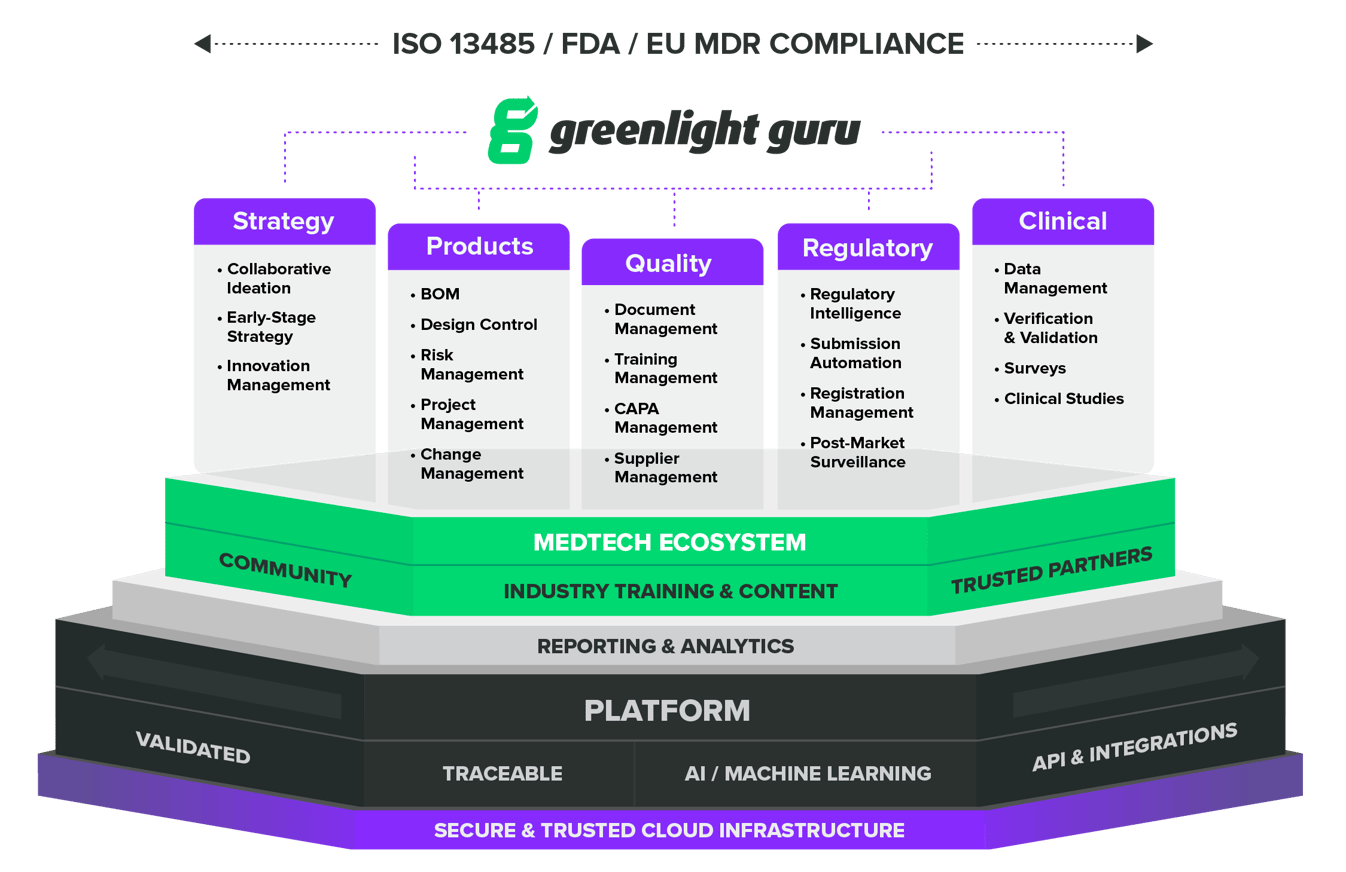
Your MedTech Lifecycle Excellence Platform
Greenlight Guru is more than your generic QMS software provider. We give you what you need – consistency and flexibility with a seamless, compliant
source of truth.
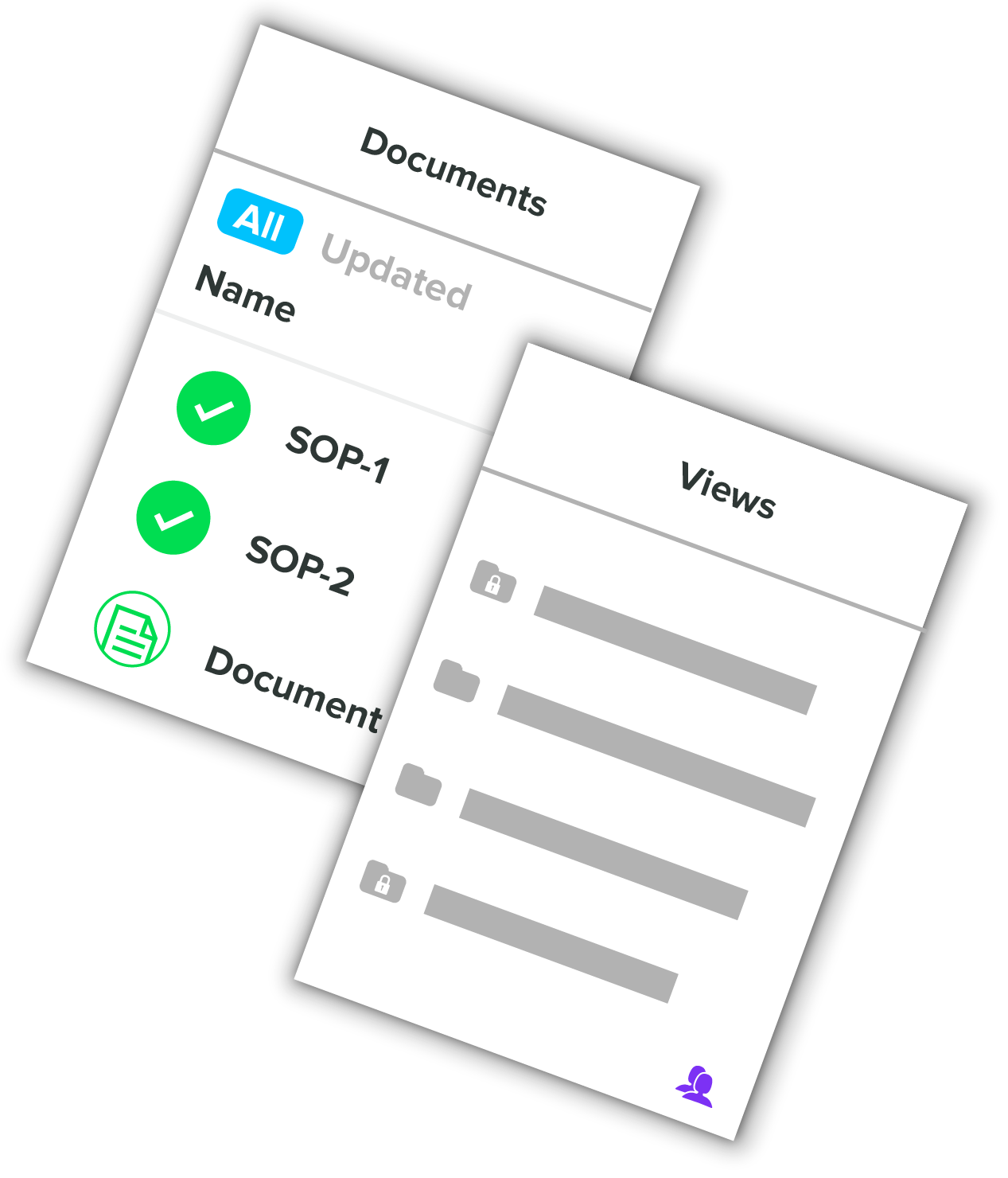


Document Management
Leverage our secure, cloud-based document management system to give your team the tools they need to succeed. Review & approve with Part 11 compliant e-signatures. Experience flexible review & approval workflows with full document revision control. Discover what’s possible with real traceability.

Change Management
Conducting changes within compliance shouldn't be a worry. Leave fretting over how to manage change control processes in the past. No matter how big the change, discover our easy-to-use, formalized change control solution that is efficient, consistent, and intelligent. Adopt formal workflows to manage, analyze, and predict change impact and revisions.

Risk Management
Experience integrated risk management, aligned with ISO 14971:2019. Greenlight Guru reduces the stress of audits and inspections by integrating risk-based thinking into your entire quality ecosystem.

Multi-level Design Controls
The new era of design control is here. Move forward with multi-level design control software that keeps innovation, quality, and traceability at the forefront. Drive collaboration for design actions, link documents, conduct project reviews, and generate a design history file — all with a single click.

Product Management
Experience the only product management software built for MedTech. Build and manage items and multi-level BOMs as you design your medical devices. Create a seamless flow from DHF to product development processes, managing critical components of your device master record (DMR) while meeting ISO 13485 requirements.

Quality Process Management
The only Quality Management Software designed to help MedTech teams deliver innovations to market, streamline compliance, and focus on quality — all in one end-to-end platform. Easily access up to date documents, maintain device traceability, mitigate risk, and build your bill of materials. Get automated quality workflows including Audit, CAPA, Nonconformance, and Complaints management.

Training Management
Eliminate the disconnect between documentation and people. Manage training with auto-generated records at the individual and document level. Ensure compliance and competency within your organization. Track training and obtain sign-offs — all in a single cloud-based solution.

AI/ML Advantages
React. Predict. See how AI and ML can transform your document and change management processes. Experience visual traceability paired with AI-powered change impact analysis. Proactively evaluate quality performance and predict issues before they happen.
Key Solution Features
While our end-to-end system covers a wide range of your business’ needs, we’ve identified five key areas that often overwhelm medical technology companies.
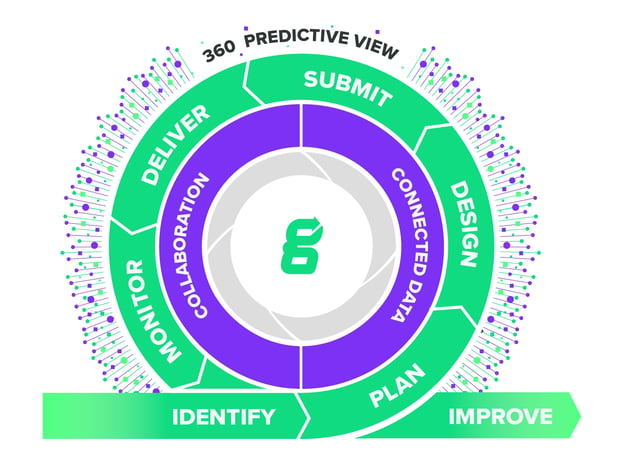
End-to-end Support Throughout the MedTech Lifecycle
Discover how our industry-dedicated platform accelerates the delivery of life-changing medical devices to all of humanity throughout your product’s lifecycle.
Identify
Plan
Design
Submit
Deliver
Monitor
Improve
Are You Ready to Experience the Greenlight Guru Difference?
With solutions to improve every stage of your product lifecycle and a team excited to support you, achieving MedTech Lifecycle Excellence has never been easier.
1000 +
trusted by medical device companies1700 +
510(k) clearances and CE markings2000 +
ISO 13485 certifications1000 +
other approvals and audits passedMore Than Software to Fill the Gaps
Even with great software, MedTech companies fail every day. Succeed with Guru Edge. Whether you’re looking for advice from like-minded professionals or researching the latest MedTech trends, we have you covered with comprehensive, industry-specific services, tools, and education.