Medical Device Risk Management Software
Integrated and Compliant Risk Management
Greenlight Guru eases audit stress with a Risk Management workspace offering full traceability with the rest of your QMS. Seamlessly integrate risk-based thinking across your device ecosystem for effortless compliance with ISO 14971:2019 and ISO 13485:2016.

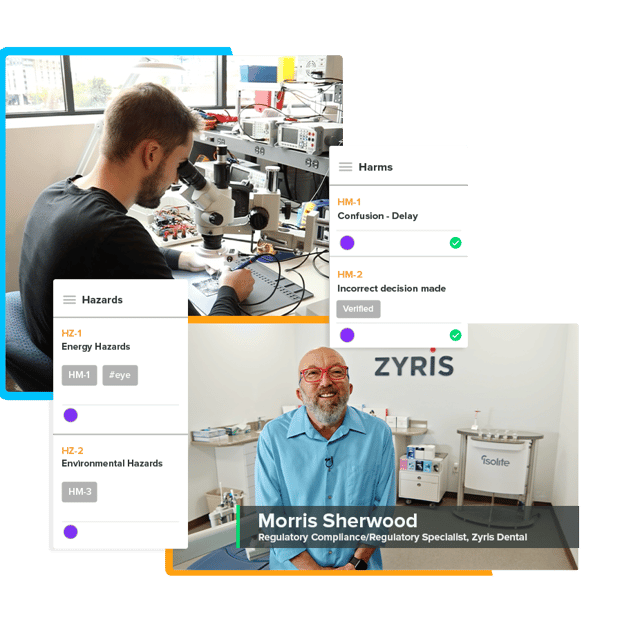
A Risk You Don’t Have To Accept
Risk management can be a difficult process. Regulations like ISO 14971:2019 make it easier for MedTech companies.
Too many companies find themselves falling short and wasting time on connecting their design controls with risk management or ensuring that risk management is a living process throughout the entire product lifecycle. For all these reasons, we developed Greenlight Guru.
The Only ISO 14971 Aligned Risk Management Software For MedTech
Leverage Greenlight Guru’s Risk Management Software to integrate risk throughout the entire product lifecycle and ensure compliance.
Effective Risk Management Doesn’t Need To Be Complex
Designed specifically for the MedTech industry with simplicity and flexibility in mind.
Link and connect design controls and documents as risk control measures to mitigate and reduce risks throughout the entire device lifecycle.
Link and connect design controls and documents as risk control measures to mitigate and reduce risks throughout the entire device lifecycle.
Build and manage your Risk Matrices with more ease and clarity than ever before.
Build and manage your Risk Matrices with more ease and clarity than ever before.
Leverage configured extensive list of International Medical Device Regulators Forum (IMDRF) codes to document hazards and patient harms. Easily configure and update the list to best suit your needs.
Leverage configured extensive list of International Medical Device Regulators Forum (IMDRF) codes to document hazards and patient harms. Easily configure and update the list to best suit your needs.
Streamline risk reviews and easily trace documentation throughout your design and development process, crafting a robust, audit-ready Risk Management File with ease.
Streamline risk reviews and easily trace documentation throughout your design and development process, crafting a robust, audit-ready Risk Management File with ease.
Ensure consistent classification of risk across the risk project. Risk levels are then evaluated based on your set criteria.
Ensure consistent classification of risk across the risk project. Risk levels are then evaluated based on your set criteria.
Benefits for Teams
For Product
- Improve the quality, safety, and effectiveness of your medical devices
- Establish connections between design controls and risk management
- Demonstrate a risk-based approach to design with full traceability to related design controls and components
Benefits for Teams
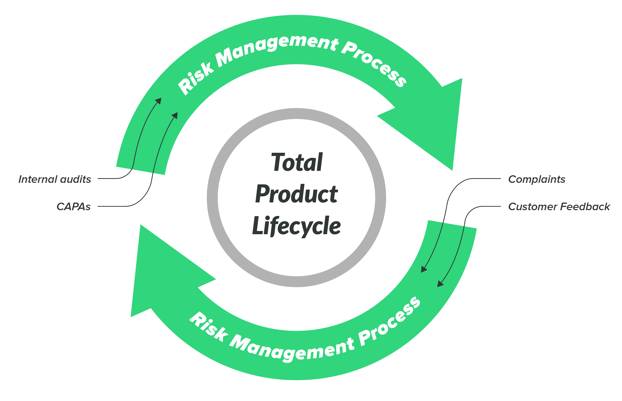
Integrate Risk-based Thinking Into Your Entire Quality Ecosystem
Risk management is a total product lifecycle process. When a project is complete and in production, keep your risk management file up-to-date throughout the entire lifecycle by electronically reviewing, signing, and approving documentation with a single source of truth.
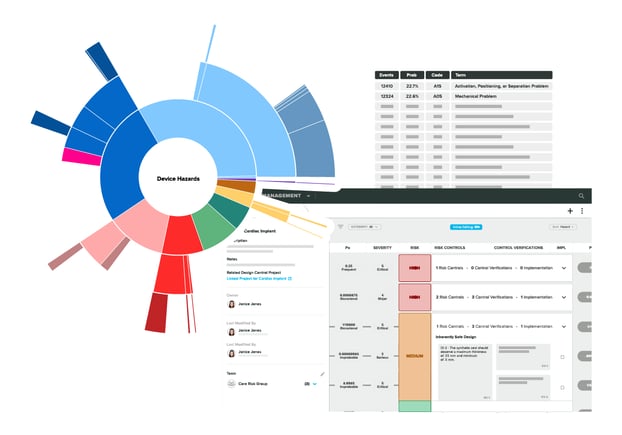
Intelligence Meets Compliance
Work more efficiently, enhance risk assessments, and keep patient safety at the heart of what you do with our first-of-its-kind technology.
Risk Intelligence gives you AI-powered insights to help you identify the most relevant device hazards and patient harms with real probabilities and severities for your devices based on real-world adverse event data.
Learn More















