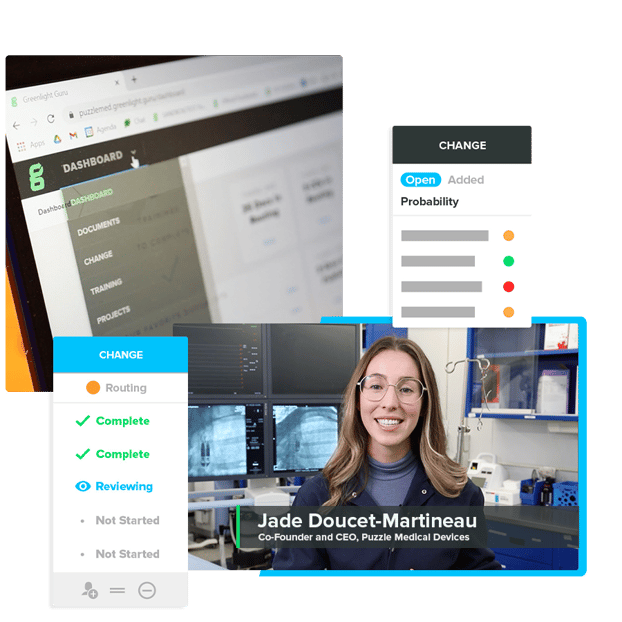
Conducting Changes Within Compliance Shouldn’t Be a Worry
The FDA, EU MDR, and ISO 13485 all require the documentation and management of change be done to standard. That’s why it’s critical to have a QMS that supports you in achieving regulatory compliance while you are developing your products and processes. We understand it’s a challenge to keep change both streamlined for efficiency and thorough enough to meet all the requirements.
Consistent & Efficient Change Management
Greenlight Guru's Change Management system enables both small and large scale change
as you need it.
Catch the Impact of Change
Designed specifically for the MedTech industry with simplicity and flexibility in mind.
Easily capture e-signatures across your teams.
Easily capture e-signatures across your teams.
All data captured from the change is generated into a document with documents impacted, approvers, approval date, effective date, signatures, and descriptions.
All data captured from the change is generated into a document with documents impacted, approvers, approval date, effective date, signatures, and descriptions.
Reminder email correspondence generated by Greenlight Guru keeps you on track with your change orders.
Reminder email correspondence generated by Greenlight Guru keeps you on track with your change orders.
Benefits for Teams
For Development Teams
Collaborate on changes that impact your development and design
- Easily review your evolving design and processes
- Approve or reject change orders as needed
- Evaluate and validate the need for any change

















