Compliant Document Management Meets Modern Workflows
Documents can quite literally pile up in both the MedTech and regulatory spaces. Leverage our secure, cloud-based document management system to give your team the tools they need to succeed. Finding, revising, and signing off on documents should never be a blocker. Discover what’s possible with real traceability.

Paperwork?
What Paperwork?
The MedTech space can be challenging, to say the least. In this ever-changing field, your processes, devices, and documents undergo multiple revisions that generate the need for new ones. It’s an endless cycle. Being able to access up-to-date versions of documents and having them on hand at a moment's notice is the key to success.
Seamless Document Management
Leverage the power of traceability with Greenlight Guru Document Management.
See the Impact of Automated Document Management
Designed specifically for the MedTech Industry with simplicity and flexibility in mind.
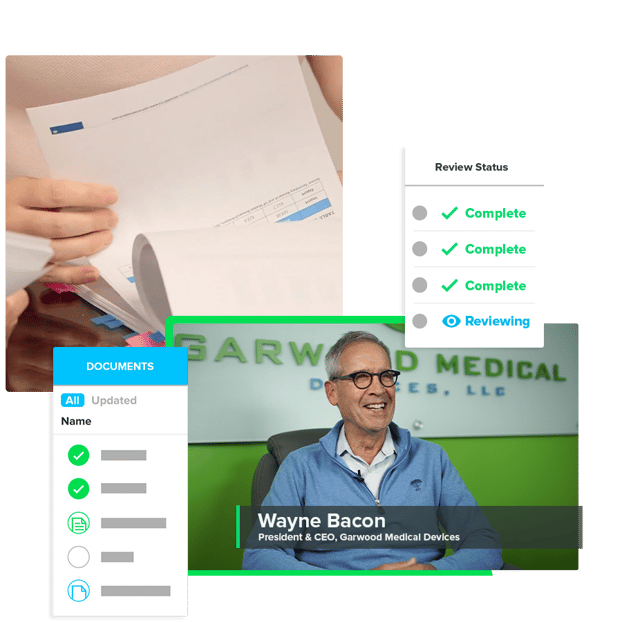
Get Part 11 compliant signatures for each of your documents so you’re always audit-ready.
Get Part 11 compliant signatures for each of your documents so you’re always audit-ready.
Documents change over time. Let’s not complicate the process.
Documents change over time. Let’s not complicate the process.
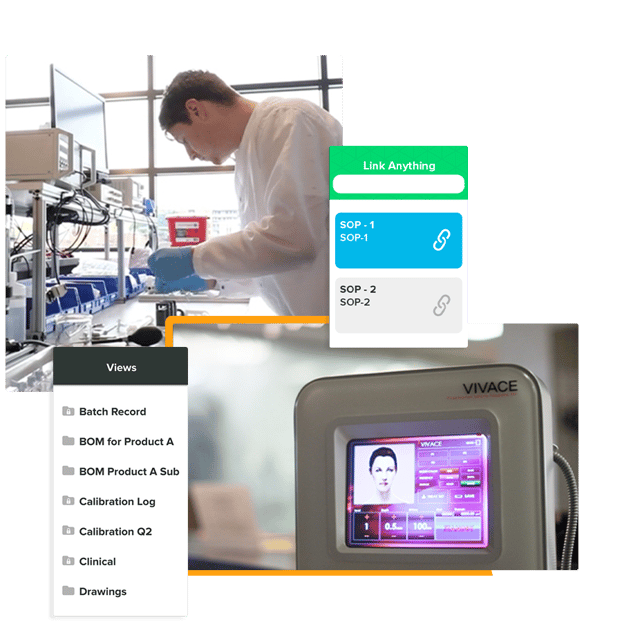
A true end-to-end software solution that enables you to link documents to your quality and development activities.
A true end-to-end software solution that enables you to link documents to your quality and development activities.
Share your views or keep them private. All it takes is a few clicks.
Share your views or keep them private. All it takes is a few clicks.
Benefits for Teams
For Development Teams
Collaborate on design and development documents
- Easily access your latest design documents
- Link documents to your design control and development activities
- House your work instructions, process validations, SOPs, and lab test methods