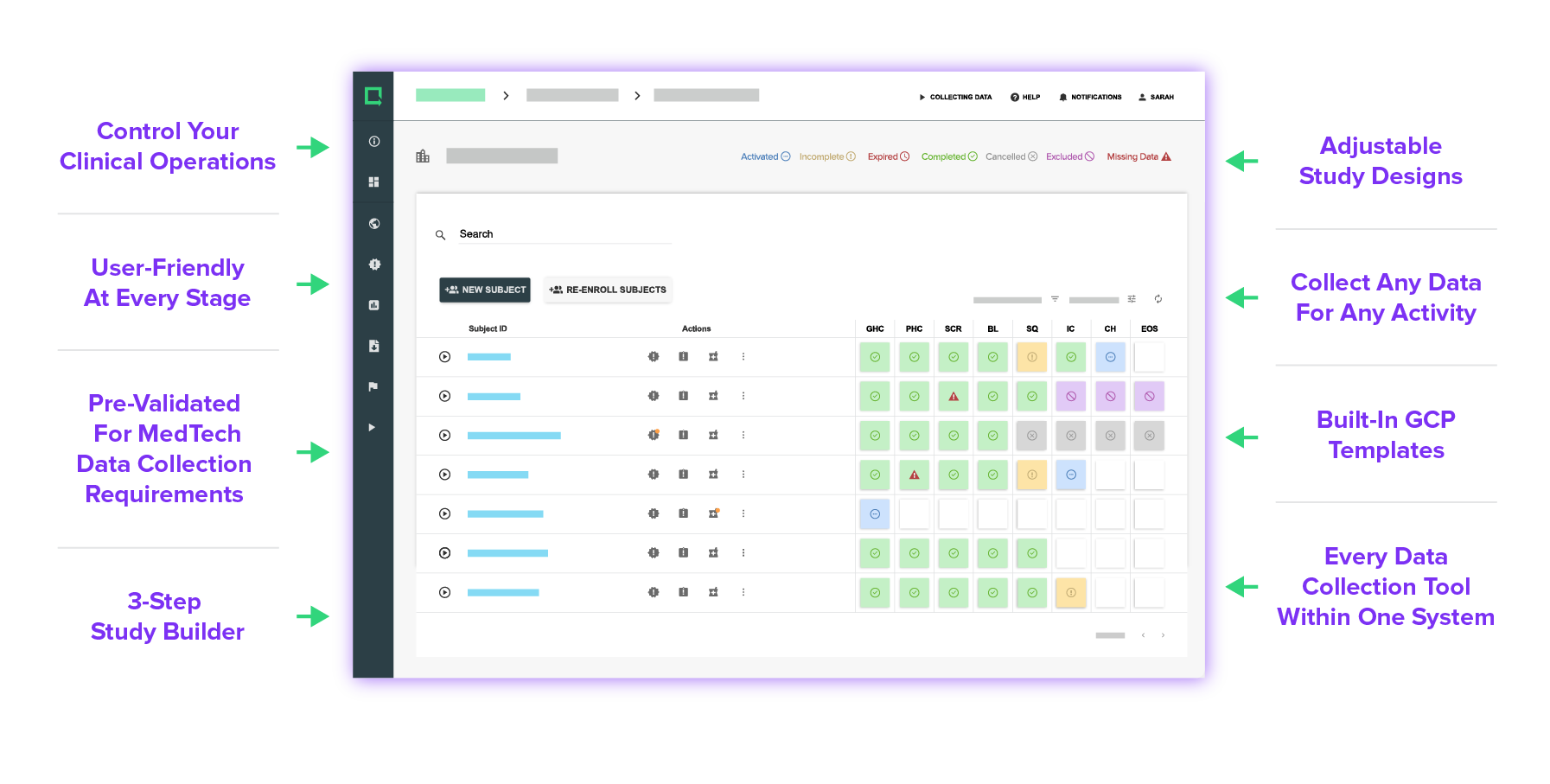
Every Feature Made for MedTech
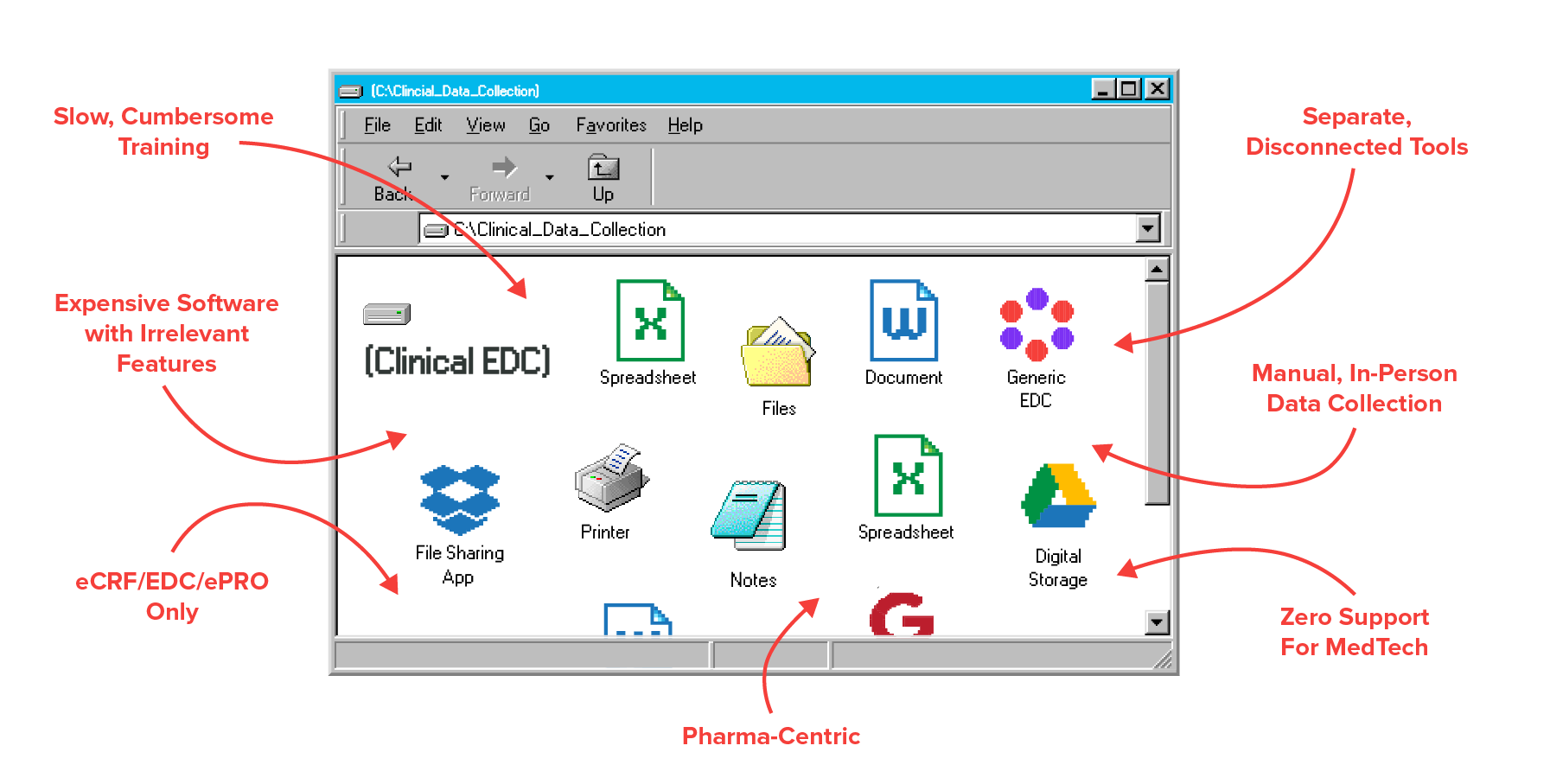
Your software is already optimized for medical device studies. That means no coding, no stressful setup, and no expensive pharma-centric features you'll never use.
Ensure compliance from the start with built-in ISO 14155:2020, EU MDR, and FDA requirements. In addition, you'll get 13 ready-to-use and customizable compliance document templates.
Easily make adjustments to your study designs according to different countries' requirements and EC approvals.
If your study subjects require one or many treatments with your device, you can use multiple activations for any visit event.
Set up a study in as little as 90 seconds with intuitive drag-and-drop elements, form validation, and customizable protocol designs. Adjust and reuse your studies as much as you need to.
Functionality Meets Convenience
Experience versatile tools and customizations that anyone can use and build your study exactly as you need it.
Design your eCRF based on your unique study requirements — simple or complex, there are no limits.
Learn MoreCreate a user-friendly experience for study subjects and increase response rates, adherence, and quality of data.
Out-of-the-box GCP-compliant surveys. Collect data with dynamic surveys to comply with EU MDR PMCF or FDA Post-Approval requirements.
Learn MoreThe mobile-first case studies series data collection tool you've always wanted. Clinician-friendly and compliant. Enable ad-hoc data entry of high-quality patient data in post-market settings.
Learn MoreFrom eConsent to randomization, custom study dashboards, and external system integrations.
Learn MoreHelping MedTech Companies
Make an Impact
See how some of the world's most advanced medical device companies use
Greenlight Guru Clinical.
No results
Frequently Asked Questions
How does EDC software improve data accuracy and efficiency in clinical research?
Data captured on paper or in generic software like Excel must then be transcribed into a database—a time-consuming task that creates the potential for human error. EDC software allows study personnel and participants to enter data directly into the electronic system, removing the need for transcription and speeding up the entire process. EDC software also provides data entry controls that help ensure fields on a form are not missed or that only appropriate values may be entered.
Read more: Electronic Data Capture Systems vs. Paper-based Data Collection
What are the primary features to look for in an EDC system?
A well-designed EDC system should facilitate compliance with relevant regulations and industry standards related to Good Clinical Practice (GCP), like ISO 14155:2020. It should have a modern, intuitive interface and allow users to collect clinical data from any source, using eCRF, ePRO, post-market surveys, and GCP-compliant ad hoc data collection.
Read more: 10 Tips for Selecting the Right EDC Software for Clinical Investigations
How does EDC software help ensure regulatory compliance?
EDC software that comes validated to industry regulations and standards will provide internal guidelines that help ensure your forms and study setup are compliant. Security features such as audit trails and permission-based access will keep users compliant with regulatory requirements for data storage and management.
Read more: Electronic Data Capture (EDC) Systems: What to Know
How secure is EDC software for storing sensitive clinical data?
Any good EDC software will come with strong security and data privacy measures for storing, managing, and transferring clinical data. For instance, Greenlight Guru Clinical uses features like continuous data backup, two-factor authentication, data encryption, permission-based access, and audit-logging to ensure the security and privacy of clinical data per regulations such as EU GDPR, HIPAA, and 21 CFR Part 11.
Read more: How to Comply with HIPAA and EU GDPR in Medical Device Studies
What integration capabilities does Greenlight Guru's EDC software offer with other systems or tools commonly used in clinical research?
Greenlight Guru Clinical’s API supports two-way communication to push and pull data directly to and from your study. Our API integrates with third-party applications, supports multiple devices, and allows for fast and secure transfer of any data into the study of your choice.
Read more: How to Switch from Paper to EDC Systems in Your Next Clinical Study
What are the benefits of using role-based access control in EDC systems?
Role-based access control (RBAC) ensures that study personnel can access only the data and tools necessary for their specific responsibilities. This minimizes the risk of unauthorized data access, enhances security, and supports compliance with regulations like ISO 14155:2020 and EU GDPR. By tailoring access permissions, RBAC helps maintain data integrity and fosters accountability across study teams.
Read more: How Technology Can Support Clinical Trial Continuity & Data Integrity
How does Greenlight Guru Clinical handle multi-language support for global studies?
Greenlight Guru Clinical supports multi-language studies by enabling localized eCRFs, ePROs, and other data collection tools in more than 40 languages. This ensures participant comprehension, improves data quality, and allows clinical teams to manage studies seamlessly across multiple locations. With customizable translation workflows, our system helps streamline global study operations.
Read more: Decentralized Clinical Trials: Are Remote Elements Right for Your Next Study?
Can Greenlight Guru Clinical manage concurrent studies effectively?
Yes, Greenlight Guru Clinical is designed to support the management of multiple studies simultaneously. Its intuitive dashboard allows clinical teams to monitor the status, data, and compliance metrics of each study in real-time. This concurrent management capability reduces administrative overhead and helps ensure all studies progress efficiently without overlap or confusion.
Read more: Boost Efficiency and Decision-Making with Enhanced Clinical Data Reporting
How does real-time data tracking in EDC systems benefit clinical studies?
Real-time data tracking provides immediate access to study metrics, participant progress, and data trends. This visibility allows teams to identify issues early, such as low enrollment rates or incomplete data fields, and take corrective actions quickly. Greenlight Guru Clinical’s real-time tracking helps sponsors and CROs maintain study timelines and optimize data quality.
Read more: How Electronic Data Capture is Transforming the MedTech Industry
Why is ISO 14155:2020 compliance important for EDC systems in medical device studies?
ISO 14155:2020 sets the global standard for Good Clinical Practice (GCP) in medical device clinical investigations. EDC systems that comply with ISO 14155:2020 ensure that data collection, monitoring, and reporting align with these rigorous guidelines. This compliance reduces regulatory risks, helps ensure ethical study conduct, and facilitates smooth submission processes for device approvals.
Read more: 15 Reasons Why Medical Device Companies Choose Greenlight Guru