How to Switch from Paper to EDC Systems in Your Next Clinical Study
.png?width=800&height=400&name=How%20to%20Switch%20from%20Paper%20to%20EDC%20Systems%20in%20Your%20Next%20Clinical%20Study%20(new).png)
Clinical trials are a crucial aspect of the medical device industry, as they are used to test and validate the safety and efficacy of new treatments and devices. However, the traditional method of using paper forms and Excel spreadsheets to manually collect and manage data in these trials is outdated and prone to human error and missing data. Even worse, this approach can compromise the accuracy and validity of the trial, making it difficult to obtain reliable results.
Electronic data capture (EDC) systems have been designed to overcome these challenges. EDC systems allow for real-time data collection and management, reducing the risk of errors and missing data. They also provide a centralized location for data storage, making it easier to manage and analyze the collected information.
And choosing an EDC system that’s purpose-built for MedTech means a solution that is highly flexible, allowing you to customize the data collection process to fit the specific needs of your clinical trial.
Let’s take a look at the process of switching from paper to EDC systems in your next clinical trial with a step-by-step guide on how to make the transition.
Prepare for a new electronic data capture system
Before implementing an EDC system in your clinical trial, there are several steps you should take to ensure a smooth transition. Begin by identifying the specific data collection needs of your trial. This involves evaluating the type of information that needs to be collected and the processes involved in collecting and managing the data.
EDC systems that are purpose-built for the medical device industry are flexible, and should be able to capture data such as:
- Patient characteristics and demographic data,
- Clinical study site and patient’s treatment group,
- Patient health status, history, and vital sign measurements,
- Treatment effects/use,
- Patient lab reports and test results,
- Readings from patient-attached medical devices (e.g. blood pressure, heart rate, oxygen saturation, blood glucose level, etc.)
Once you have a clear understanding of your data collection needs, you can begin researching and selecting an appropriate EDC system. There are a variety of EDC systems available, so it is important to choose one that fits your clinical trial's requirements and budget.
You should also make sure that your EDC solution is built with compliance needs in mind. All clinical investigations of medical devices must be carried out in accordance with ISO 14155:2020. The best EDC systems built for MedTech will be validated out-of-the-box per ISO 14155. They may also include customizable regulatory compliance templates, like ISO 14155:2020 and EU MDR-compliant AE reporting.
The next step is to ensure that all individuals who will be using the EDC system receive adequate training on its use. Be sure you’re considering the scope of the clinical trial, and how many different individuals at different sites for data collection there may be, such as at different care clinics, labs, or other healthcare facilities.
Doing so will require a number of procedures which describe how you organize your clinical operations, such as SOPs and quality standards. Not only do these procedures need to be in-place prior to training, they also need to be created for your EDC system. Any delays in generating these procedures could cost you time and resources, as well as severely limiting your trial’s sample size.
Thankfully, with our deep experience in both medical devices and clinical data collection, Greenlight Guru Clinical (formerly known as SMART-TRIAL) offers customizable templates specifically designed to facilitate those procedures.
Implement the electronic data capture system
Once you have selected and prepared for an EDC system, it is time to implement it in your clinical trial. The first step is to set up and configure the EDC system specifically for your needs. This involves creating an account and defining the parameters for data collection, such as the types of data that will be collected and the forms that will be used.
Because each clinical study is different, data collection practices vary significantly between clinical studies. EDC systems allow clinical trial sponsors to design customized eCRFs to ensure that researchers collect the necessary data as per the stated research hypothesis and data collection plan.
It is also important to thoroughly test the EDC system before the trial begins to ensure it is functioning properly. This includes testing the forms, data collection process, and clinical trial data management functions to ensure that everything is working correctly.
Remember: these forms should be user-friendly and effective in capturing the necessary information. You should also consider the design and layout of the forms, as well as the process for submitting and reviewing the data, which may vary based on where the data is being collected.
Medical device trials are, by nature, quite varied. Devices need to be studied across the entire product life cycle, from premarket submission to post-market surveillance. And as device studies change in size and purpose, you need an EDC system that can grow along with it.
With Greenlight Guru Clinical, you get a full clinical toolbox that allows you to add new modules and eCRFs, enabling you to track the clinical data you need at any time.
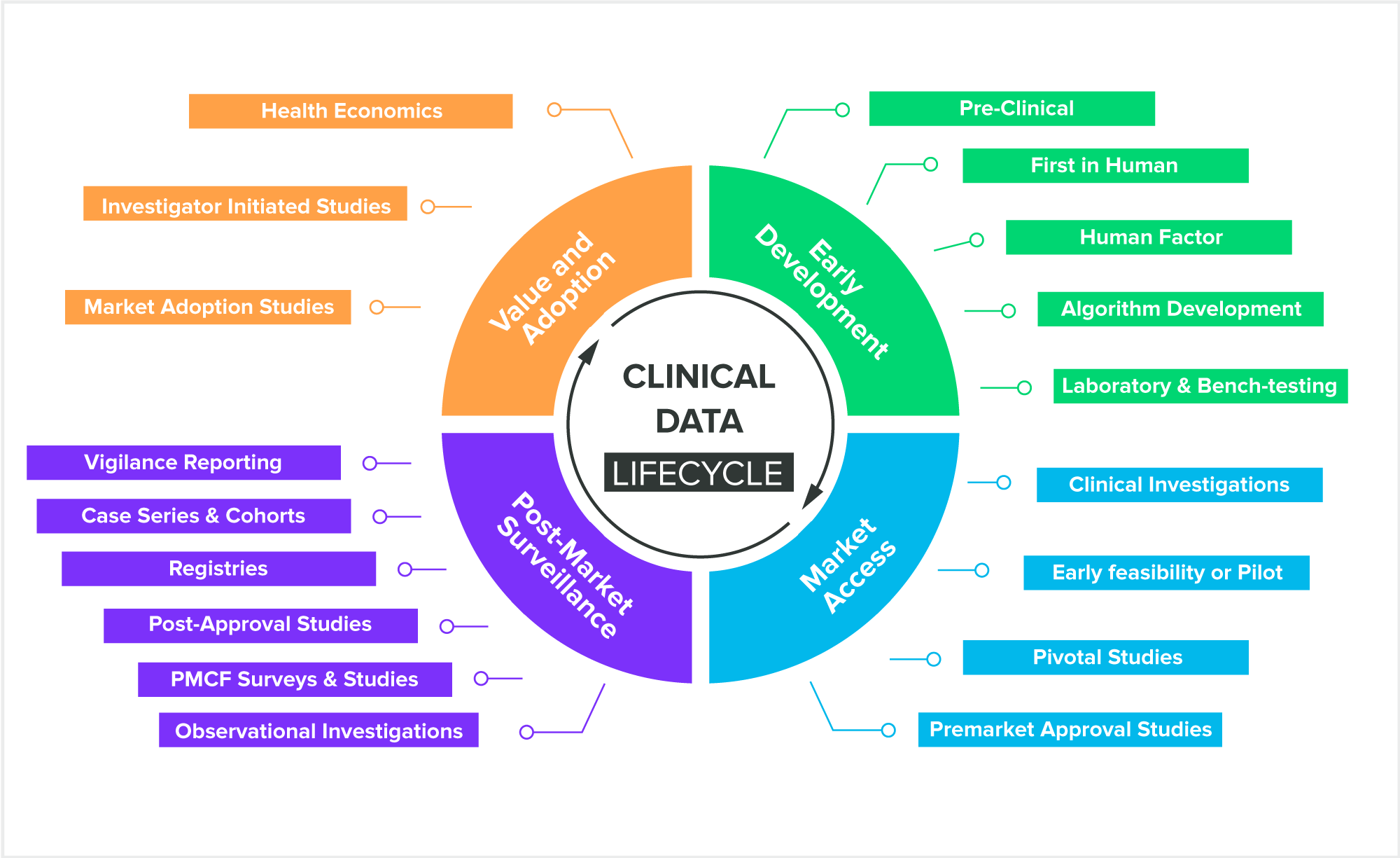 Clinical Data Activities Across Product Lifecycle
Clinical Data Activities Across Product Lifecycle
Manage electronic data capture in your clinical trial
Now that you’ve implemented your new EDC system, trained your team, and designed your optimal eCRFs, it’s time to start capturing the data in your clinical trial. EDC systems provide real-time monitoring of data collection, allowing you to see how the study is progressing and identify any issues quickly.
There are three methods for inputting the data to an EDC system during a clinical trial.
- Direct Data Entry. The first option for EDC data collection is direct data entry. Researchers can login to the EDC software using secure access credentials, open the relevant eCRF for the study, and enter clinical data into the system where it immediately becomes available for data reviewers and other stakeholders.
- Transcription from Paper or Electronic Sources. Researchers can also choose to complete CRF forms the traditional way, using paper, then transcribe those paper CRFs into the EDC system at a later time. Transcription-based data collection may also take place when data from patient-reported outcomes (PRO) or electronic health records (EHR) is entered into the EDC system.
- Automatic Transmission. An important advantage of using electronic data capture (EDC) instead of paper-based data collection is the ability to capture data via automatic transmission. Modern EDC systems can receive data transmissions from ePRO instruments (which are sometimes a part of the EDC software system), and connected medical devices (DHT)s, automating those portions of the data collection process.
Another important aspect of collecting and managing data is the use of electronic signatures. These can save time and improve the accuracy of data collection, as they allow study participants to sign forms and consent forms electronically, reducing the risk of errors and improving the speed of data collection.
However, you’ll want to be absolutely sure that the EDC system you choose has e-signatures that are compliant with FDA's 21 CFR Part 11 and the EU General Data Protection Regulation (GDPR).
Maintain and optimize your EDC system for future clinical trials
With your EDC system up and running, it’s still important to perform routine maintenance and optimization, all as a way of ensuring that data is being collected accurately, efficiently, and securely.
Remember that while EDC systems can help reduce errors, it's still important to have a robust quality control process in place. This should include regular audits of data to ensure that it's accurate and complete.
Here are some best practices and suggestions to keep in mind:
- Regular maintenance is critical to ensuring the EDC system continues to function properly, and includes software updates, backups, and system checks.
- Continued user training is critical to ensure that they are using the EDC system effectively and efficiently.
- Regular audits of data can help to identify any issues or discrepancies in the EDC system, and can help to ensure that the data collected is accurate and complete.
You should also be readily on the lookout for where to make any future Improvements. This might mean implementing new technologies or integrating with other software and devices.
And lastly, you should also be aware of any new updates to regulations that apply to clinical studies, as compliance with these should be a top priority for your medical device during post-market studies.
Make the switch simple with Greenlight Guru Clinical
If you’re ready to get serious about your clinical data collection, it’s time to check out Greenlight Guru Clinical, the first and only data gathering and management system that’s purpose-built for medical devices and diagnostics.
With Greenlight Guru Clinical, medical device companies can create customized eCRF forms and digitize data collection for clinical investigations, in-human studies, and post-market surveillance activities. It’s also out-of-the-box compliant with ISO 14155:2020 and includes templates for data collection planning and regulatory compliance that help medical device companies execute organized, cost-effective, and compliant studies that bring products to market faster.
Ready to learn more? Contact us today for your free personalized demo of Greenlight Guru Clinical.
Jón Ingi Bergsteinsson, M.Sc. in Biomedical Engineering, is the co-founder of Greenlight Guru Clinical (formerly SMART-TRIAL). He was also the technical founder of Greenlight Guru Clinical where he paved the way for the platform’s quality standards, data security, and compliance.
Read More Posts
QMSR Mythbusters Episode
The “just enough” QMS: what does your pre-market team really need?
Did Christmas come early to the EU? Proposal to simplify EUMDR
Get your free Efficient Study Setup checklist
Tips for Clinical Data Collection and Study Setup











