How to Calculate Sample Size for Medical Device Studies
.png?width=800&height=400&name=The%20Medical%20Device%20Sample%20Size%20Cookbook%20(new).png)
Sample size has always been one of the first design choices a medical device sponsor has to make for a clinical study. But the calculation of sample size is not the most straightforward process and often leaves clinical teams wondering which method to use.
As an Electronic Data Capture vendor for medical device companies, we are experiencing greater interest in EDC for PMCF studies and surveys. But we’re also seeing a major increase in requests for feedback on various study design choices - like for example:
What is the appropriate sample size for my medical device study/survey?
How can I provide justification for sample size in a medical device study?
Can I use a sample size calculator for my clinical trial?
This blog post will provide insights on all of these questions and offer in-depth advice on how to calculate sample size in medical device clinical studies. This is a sneak peek of The Medical Device Sample Size Cookbook.
NOTE: We always recommend avoiding generic sample size calculators for clinical trials, as the many variables between different medical device studies vastly increase calculation uncertainty. In addition, medical device sample size justification may be negatively impacted by utilizing simplified tools for sample size calculations.
What is sample size in medical device studies and why should you care about it?
In most cases, it’s impossible to conduct a medical device clinical study that includes the total population of interest (i.e. target population), especially if the population size is indefinite. Instead, we conduct studies with a sample from the population, in the hope that the information gathered about the sample will enable us to make inferences about the total population.
So, sample size calculation in medical device studies is actually the process of determining what is the minimum number of samples that would make a study's outcome/s statistically significant (make your results more reliable in statistical terms).
To calculate the sample size for a clinical study, we use statistical equations that employ inputs that mirror the population(s), study objective and design. But the problem with the calculation is that it is based on assumptions on these inputs, and not necessarily the ‘best’ or ‘correct’ values. Therefore, the calculation is only as good as the assumptions you make.
If your assumptions are too far off you might end up with a too small or too large sample size, which may affect the study outcomes and conclusions. Thus, to minimize the likelihood of your calculation returning a too-small or too-large sample size, your assumptions must be evaluated thoroughly.
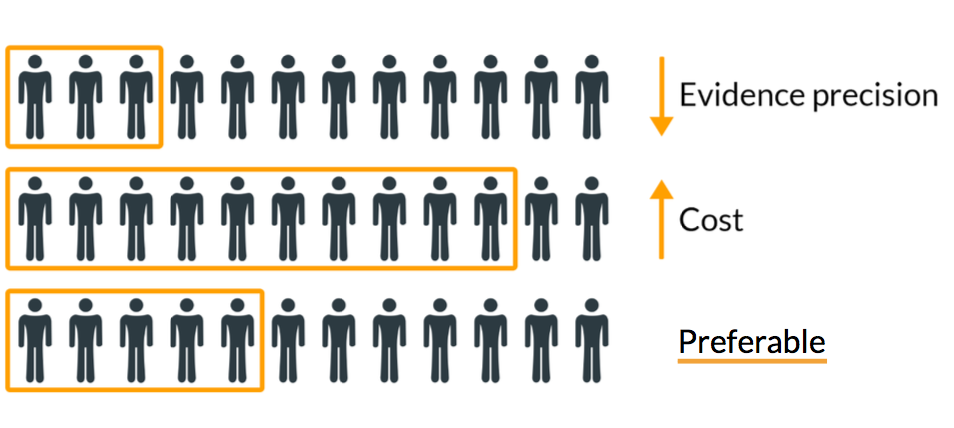
Best practice for medical device sample size calculation
With the appropriate sample size calculation for a medical device study you can:
-
Increase the likelihood of study results to mirror a true effect (or difference)
Your clinical study results are more likely to portray the reality, rather than just chance alone. -
Limit unnecessary exposure
From an ethical and risk-based standpoint you should only include the necessary number of subjects in your study. -
Improve precision and reduce cost
The sample size is highly correlated with the cost of operation and the precision of your clinical evidence. With the appropriate calculated sample size, you ensure that you miss both evidence precision and cost.
The EU MDR describes the requirements for both a Clinical Investigation Plan (CIP) and a PMCF plan. In both cases, medical device study sponsors must document the various design choices for a clinical study, such as the choice of sample size, and provide a rationale for the appropriateness of the procedures and the methods used.
Even though you are not conducting a clinical study as a part of your PMCF plan, but a survey or a cohort of some kind, you still need to provide rationale or justification for your design choices - including the sample size.
Many ethical committees also require justification for the choice of sample size before approving a clinical study. And depending on the study type, and the market approval pathway in the United States, the FDA requires justification of the sample size calculations as a part of a statistical analysis plan as well.
Many fail to realize that it is the combination of both the statistical and clinical assumptions that determines the sample size justification and thereby applicability. By leaving out either of the two, you are only providing partial justification on your sample size.
Preparing for sample size calculations in clinical research
To calculate the appropriate sample size for your study, you need to have knowledge of the expected study results. This is used to determine how the study should be conducted, what data should be collected and how its results should be analyzed (and statistically tested).
When it comes to conducting the sample size calculation, you apply statistical equations that are derived from the hypothesis you want to test. But before the equations come into play you need to keep in mind that a few other things have an impact the method of medical device sample size calculation, such as:
What is the purpose of your clinical study?
When it comes to conducting studies whose purpose is to confirm a theory or claim for clinical performance or safety, such as pivotal trials, clinical investigations for market access, or PMCF, you will need to provide justification for sample size calculation (at least according to the EU MDR).
What is the objective of your clinical study?
Every clinical study has an objective, i.e. whether you want to test your medical device's superiority, non-inferiority, equivalence, or another effect/difference. The study objective will have a direct impact on the choice of sample size calculation (the formula for sample size calculation) and your statistical hypothesis test.
Study endpoints
Depending on the nature of the endpoints in your study, sample size calculation formulas have to be adjusted, especially if the endpoint involves multiple comparisons. In some cases, you can use different types of endpoints to evaluate the same clinical outcome. But depending on the endpoint you choose, the sample size can change drastically.
Study design
Whatever design you choose, you need to know that it will impact the sample size calculation. For example, a crossover study and a parallel two-group study require two different equations to calculate the sample size, the crossover study only requires a part of what a two-group parallel study needs in terms of sample size.
Statistical tests
There are numerous different statistical tests that can be used to analyze and test the results of your study. Usually, this must be a part of the statistical analysis plan and is highly related to the endpoints you are looking to collect. Depending on the statistical tests you choose for your study, you will need to use the appropriate sample size formula, and thus extract the sample size from the formula to calculate the sample size for the study.
The basic inputs behind the sample size calculations
For most sample size calculations in clinical research, you need five basic inputs. All five inputs are values (or numbers) that you must define or make assumptions on, and depend on the study objective, endpoints, and design. Sample size calculation is very sensitive to the inputs used, which invites a large risk of imprecision or error.
Due to the nature of some of these inputs, it is rare to have an ‘accurate value’. But to minimize the imprecision, you need to carefully evaluate each input from both a clinical and statistical standpoint, using existing know-how of the domain of interest, the clinical pathway/setting and your device.
The five basic inputs for most sample size calculations are:
-
Statistical Hypothesis
-
Significance level
-
Power
-
The minimal clinically meaningful effect
-
Variability
Apart from the basic inputs, there are three other additional factors that should be taken into account when calculating sample size. Depending on the study objective and design, these factors can impact the final number of subjects needed for your study.
The three factors to consider when calculating sample size are:
-
Test Margin
-
Subject Drop-out rate
-
Treatment allocation
Final thoughts
Sample size calculation for medical device studies should not be treated lightly and there’s no ‘all-in-one’ solution to determine a clinical study sample size.
To pass the scrutiny of regulators and Notified Bodies, companies will need to provide statistical and clinical justification of the sample size calculation. It’s not enough to solely document the statistical assumptions made, but it’s the combination of both statistical and clinical (scientific) assumptions that determines the sample size calculation applicability.
Sample size justification is required for both pre-market clinical investigations and post-market activities.
Not all medical device companies have the privilege of employing a biostatistician or a data scientist in-house to assist with sample size calculation. This forces clinical teams to look for advice outside of their organization (which is also recommended).
Watch the webinar and get insights directly from a biostatistician.
Knowing this, we felt it was important to provide clinical teams with insights into the area of sample size calculation, in the hope that this could simplify the process, and better prepare for discussions with biostatisticians.
This leads us to the purpose of the e-book which is to explain the process of sample size calculation, in simple language, and exemplify how statistical methods can be used to calculate sample size for medical device studies.
If you are interested in how we help clinical teams with their clinical data management, contact us directly.
Jón Ingi Bergsteinsson, M.Sc. in Biomedical Engineering, is the co-founder of Greenlight Guru Clinical (formerly SMART-TRIAL). He was also the technical founder of Greenlight Guru Clinical where he paved the way for the platform’s quality standards, data security, and compliance.
Read More Posts
8 Tips Before Calculating Sample Size in Medical Device Clinical Studies
How to Design a Successful Post-Market Clinical Follow-Up (PMCF) Survey
The Medical Device Practical Guide to PMCF Requirements under EU MDR
Get your free eBook PDF
The Medical Device Sample Size Cookbook