Medical Device Clinical Survey Software
The Ideal Post-Market Survey Tool for MedTech
Collect scientifically valid and compliant post-market data with Greenlight Guru Clinical’s market leading data collection tool, specifically built for medical device and diagnostics post-market surveys under EU MDR and FDA.

Pre-Validated Post-Market Surveys
Don’t risk your device’s market position with flawed post-market data! Ensure optimal and compliant post-market survey data with minimum effort and resources. The Greenlight Guru Clinical Survey tool comes pre-validated per ISO 14155:2020 and enables GCP compliance out-of-the-box. All to help you set new standards for your post-market data quality.

Get your Post-Market Surveys Right!
We’ve built the ideal post-market survey tool for your device, so you can focus on improving the quality of life for your patients.
Post-Market Surveys Shouldn’t Be Hard
Designed to streamline your entire post-market clinical data collection process, minimizing time and resources needed to ensure consistent, high quality data.
Fast and intuitive 3-step study builder. Create any study within 90 seconds.
Fast and intuitive 3-step study builder. Create any study within 90 seconds.
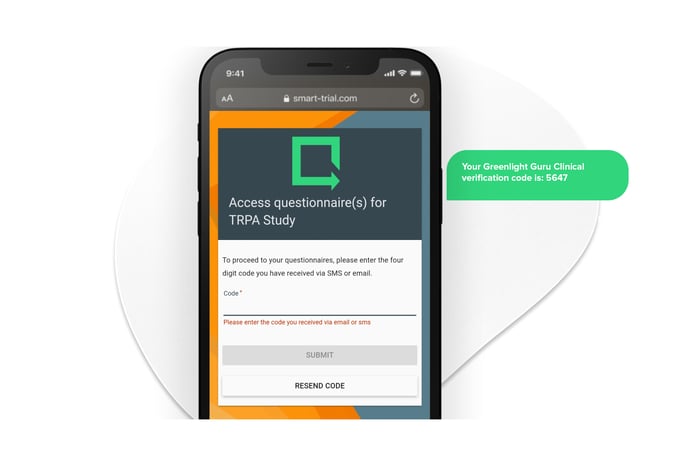
Versatile survey distribution to ensure high patient engagement.
Versatile survey distribution to ensure high patient engagement.
Enhance subject experience by using your own logo and name for all subject communication.
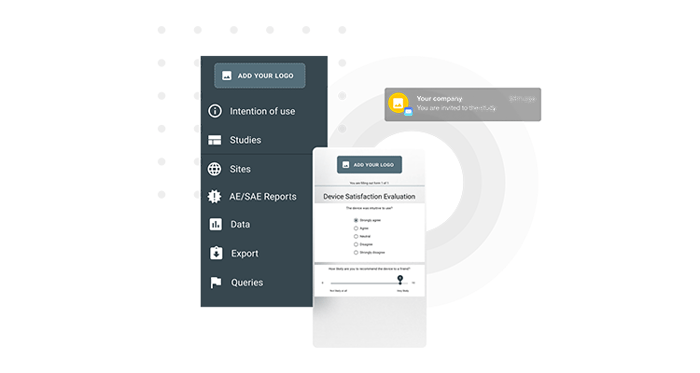
Enhance subject experience by using your own logo and name for all subject communication.