Process Verification vs Process Validation: What’s the Difference?

In a highly regulated industry like MedTech, manufacturing processes must undergo either process verification or process validation to ensure they’re consistently producing the correct result.
The question is, which one should you use?
Verification and validation are two different activities, and they’re used under different circumstances. And knowing when to validate or verify a process is essential from both a quality and regulatory perspective.
So, let’s take a look at what process verification and process validation refer to, and when you should use each of them in medical device manufacturing.
What’s the difference between process verification vs process validation?
First, I want to note that process verification and validation are not the same thing as design verification and validation. The latter are performed as part of design controls, and have their own specific definitions and steps.
To get some clarity around the difference between process verification and validation, let’s start by looking at the regulations. In this case, I’m talking about 21 CFR Part 820, otherwise known as the FDA’s Quality System Regulation (QSR).
What is process verification according to the QSR?
In 820.3(aa) of the QSR, FDA defines verification as “confirmation by examination and provision of objective evidence that specified requirements have been fulfilled.” There is no specific definition of “process verification” in the QSR, but the general verification definition can be applied to processes as well as products or services.
To verify that a process is working, you need to be able to provide some type of objective evidence—from a test or measurement, for instance—that proves the outcome of the process meets your specified requirements.
What you need to remember here is that process verification requires some sort of quantitative proof that specifications have been met. In the simplest terms, if you specified that a part should be exactly 20 mm in length, you could verify that by measuring the parts that your process produces against the specification of 20 mm.
What is process validation according to the QSR?
FDA defines process validation as “establishing by objective evidence that a process consistently produces a result or product meeting its predetermined specifications.”
At first glance, this looks very similar to the definition for verification. However, process validation does not measure or test a final product against specifications, like when you verify a process is working correctly.
In fact, in 820.75(a) of its QSR, FDA states that process validation must occur “when the results of a process cannot be fully verified by subsequent inspection and test.” In other words, when you can’t use process verification to prove your process is working as intended.
In practice, this may include processes like:
-
Sterile package sealing
-
Aseptic filling
-
Welding, soldering, painting, or heat treating
-
Injection molding
In the case of sterile packaging, for example, verifying the result would require opening it—thus destroying the sterile barrier and rendering the whole process moot.
When should you use process verification vs process validation?
With a process such as sterile packaging, the decision to use process validation instead of process verification is practically made for you.
However, not all decisions regarding process validation vs process verification are that easy to make. If you’re considering whether you need to verify or validate a process, then start with the IMDRF guidance on process validation. This document includes a flowchart that breaks down the general decision of whether to verify or validate a process.
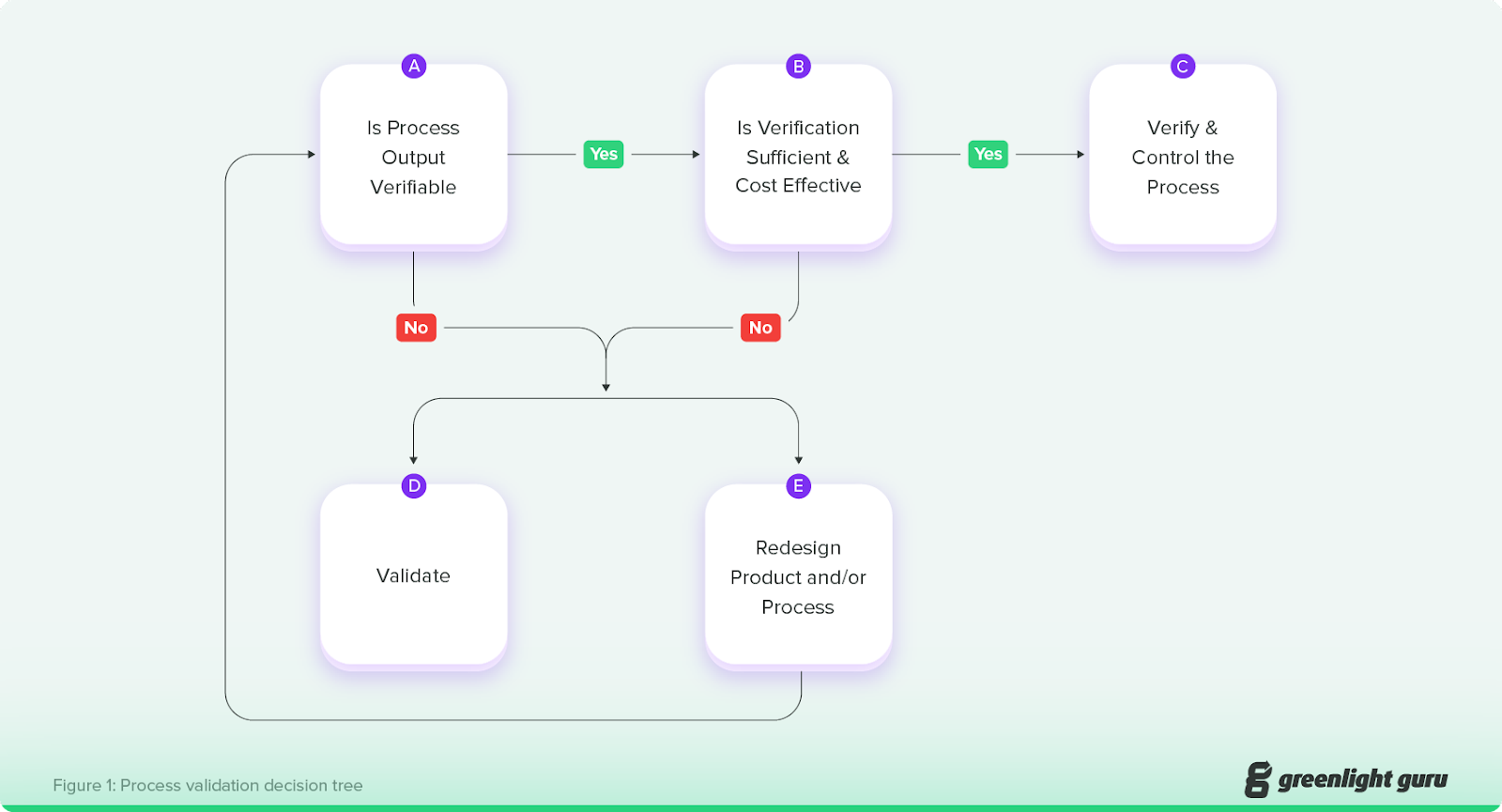
Source: GHTF Process Validation Guidance
As you can see, the decision revolves around two questions:
-
Is the process output verifiable? If not, then you should validate or redesign the product and/or process so that you can verify it.
-
Is verification sufficient and cost effective? Essentially, is verification alone sufficient to eliminate unacceptable risk and is it a cost-effective solution?
Some processes may be verifiable, but from a business perspective, it may make more sense to validate them instead. The guidance document offers these examples for processes in which you might choose validation over verification:
-
Numerical control cutting processes
-
Certain human assembly processes
-
Certain cleaning process
-
Certain filling processes
For complex manufacturing processes and sub-processes, the decision to validate or verify may be more difficult than the examples I’ve used here. Just remember that at the end of the day, you should choose the option that provides the most confidence that this process will result in a safe and effective medical device.
What are the stages of process validation?
If you do choose validation for one of your processes, you’ll then go through the three stages of process validation: IQ, OQ, and PQ, which stand for:
-
Installation Qualification (IQ) - Installation qualification is used to ensure that the installation of any necessary equipment, piping, services, or instrumentation has been executed in accordance with the manufacturer's requirements.
-
Operational Qualification (OQ) - During operational qualification, the equipment should be tested to determine process control limits, potential failure modes, action levels, and worst case scenarios.
-
Performance Qualification (PQ) - In the performance qualification phase, the goal is to demonstrate that the process will consistently produce acceptable results under normal operating conditions.
If performed correctly, IQ, OQ, and PQ should provide a high degree of assurance that your process will consistently produce the correct result.
Track all your quality activities in a single, centralized location with Greenlight Guru
As the old MedTech adage goes, “If you didn’t document it, it didn’t happen.” Part of staying audit ready at all times is knowing that activities like process verification and process validation have and you know where to find them at a moment’s notice.
At Greenlight Guru, we built our QMS platform with medical device companies just like yours in mind. Our comprehensive solution includes document management software that ensures everyone is working on the most recent version of a document—and that same document can be quickly found during audits and inspections without chasing down stakeholders or turning over filing cabinets.
So if you’re ready for a QMS that was purpose-built for medical device companies like yours, then get your free demo of Greenlight Guru →
Etienne Nichols is the Head of Industry Insights & Education at Greenlight Guru. As a Mechanical Engineer and Medical Device Guru, he specializes in simplifying complex ideas, teaching system integration, and connecting industry leaders. While hosting the Global Medical Device Podcast, Etienne has led over 200...
Related Posts
3 Common Misconceptions About Medical Device Labeling
3 Life[cycle] Hacks for Integrating Risk Management throughout all Device Phases
Introducing Adaptable Quality Processes: custom fields, enhanced visibility, and easy exports
Get your free PDF
Master Validation Plan (MVP) Form











