Greenlight Guru for QA & RA Professionals
Ensure your company delivers compliant, high-quality devices with our industry-specific end-to-end quality management system. With Greenlight Guru, you’ll be able to digitally track and manage quality events, ensure your team's documentation is audit-ready, and drive traceability throughout your processes.



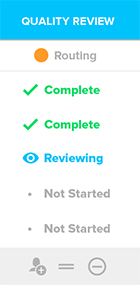
.png?width=140&height=204&name=Design_Verifications-284x412%20(1).png)
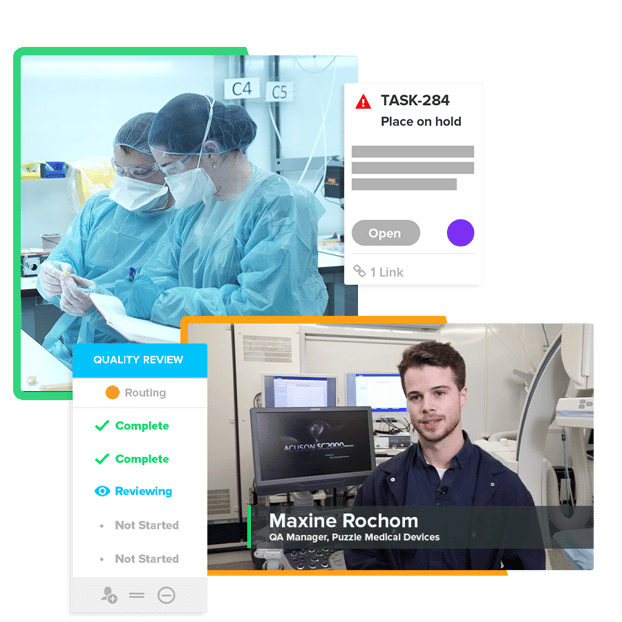
Say Goodbye to Disconnected QA and RA Processes
From trying to keep up with evolving compliance requirements to simply not having enough time, the QA and RA process can be a headache — but it doesn't have to be. When you're working with Greenlight Guru, you’ll never have to fear that you’re missing something. You’ll be able to navigate your QMS system with ease, gain visibility into historically-siloed data, provide accurate risk analyses, and keep your team’s go-to-market plan on track. See how a QMS designed specifically for MedTech QA professionals makes your life easier — you deserve it.
Why QA & RA Professionals Love Greenlight Guru

Turn Quality Into An Asset
Enable a culture of quality by managing quality events like CAPA, Customer Feedback, and Audits in Workspaces with 21 CFR Part 11 compliant review and approval workflows to collaborate with internal and external stakeholders.

Break Down Silos To Unite Your Teams
We provide a democratized approach to product development, helping organizations share information laterally across departments throughout the product life cycle. Our approach also breaks down organizational silos that restrict your department’s ability to collaborate effectively with other teams.
Discover More
Hit Tight Deadlines
Quickly organize and submit for FDA approvals or audits, ensuring no delays on go to market deadlines and satisfying both leadership and product team demands.
See for Yourself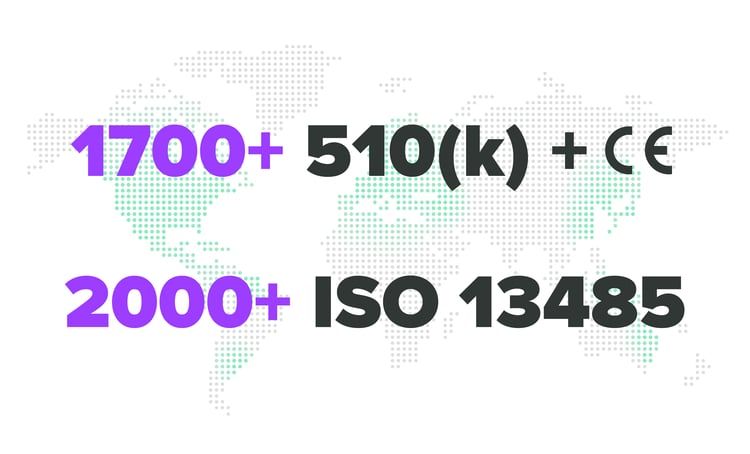
Be An MVP For Your Organization
Free your team to do more real work by consistently helping your company clear compliance milestones while creating a high-quality product that meets user needs.
See Our Software In Action
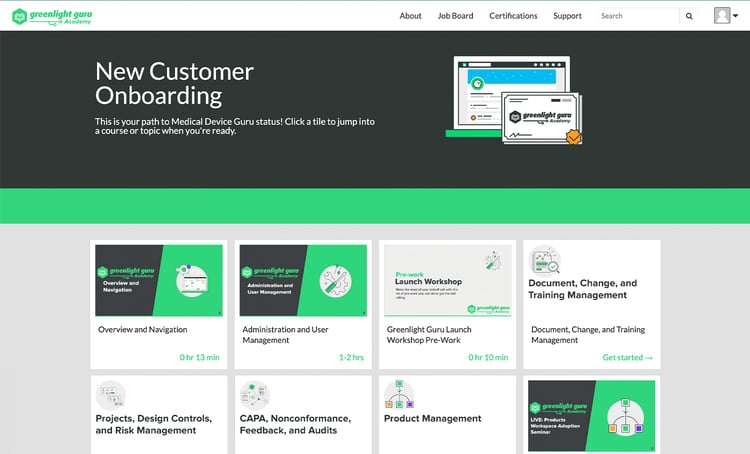
Leverage educational content from QA & RA experts
With Greenlight Guru Academy, you’ll learn practical lessons and be able to access comprehensive certifications applicable to the entire MedTech industry.
Start Learning Today
Turn Quality Into An Asset
Enable a culture of quality by managing quality events like CAPA, Customer Feedback, and Audits in Workspaces with 21 CFR Part 11 compliant review and approval workflows to collaborate with internal and external stakeholders.

Break Down Silos To Unite Your Teams
We provide a democratized approach to product development, helping organizations share information laterally across departments throughout the product life cycle. Our approach also breaks down organizational silos that restrict your department’s ability to collaborate effectively with other teams.
Discover More
Hit Tight Deadlines
Quickly organize and submit for FDA approvals or audits, ensuring no delays on go to market deadlines and satisfying both leadership and product team demands.
See for Yourself
Be An MVP For Your Organization
Free your team to do more real work by consistently helping your company clear compliance milestones while creating a high-quality product that meets user needs.
See Our Software In Action

Leverage educational content from QA & RA experts
With Greenlight Guru Academy, you’ll learn practical lessons and be able to access comprehensive certifications applicable to the entire MedTech industry.
Start Learning Today


















