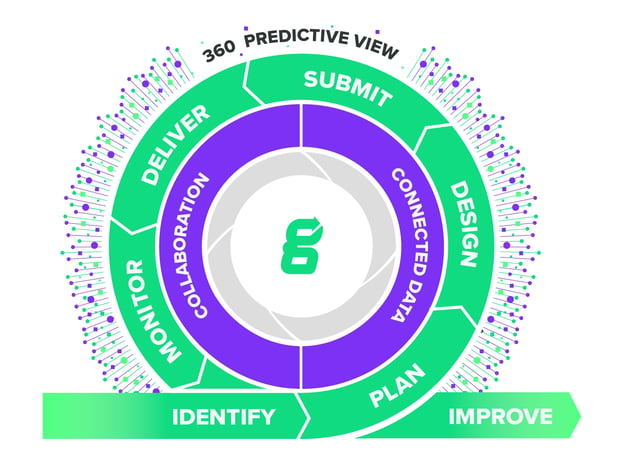
Streamline Product Development Throughout The Entire Product Lifecycle
Greenlight Guru’s single, centralized platform provides end-to-end traceability so you can drive accountability and meet deadlines like never before. This enables you to demonstrate a risk-based approach to development whether you are in early phases of design or already commercialized.
Why R&D Professionals Love Greenlight Guru
Extend your resources by reducing manual errors
Greenlight Guru offers innovative tools that focus on a paperless system, saving time and resources. With your migration away from paper and use of increased automation, you drastically reduce risk of manual error, which results in overall cost savings.
Start Saving Today
Deliver Safer Products Faster
Greenlight Guru ensures your processes align with leadership’s go-to-market timelines. Automation allows you to integrate quality and risk management into your design and development processes from the start — ensuring acceleration of your overall timeline.
Explore Product Development
Gain new visibility and mitigate risk
Incorporate risk controls into the product development process with the only cloud-based risk management solution that aligns with ISO 14971:2019. Demonstrate a risk-based approach to design with full traceability to related Design Controls and Components.
Learn More About Risk
Automatically maintain full traceability
Easily manage multi-level device requirements in your traceability matrix and generate a completed Design History File on demand. Create detailed design control objects, link complex configurations, and attach documents with a single click.
Streamline Traceability
Leverage educational content from product development experts
With Greenlight Guru Academy, you’ll learn practical lessons and be able to access comprehensive certifications applicable to the entire MedTech industry.
Start Learning Today
Extend your resources by reducing manual errors
Greenlight Guru offers innovative tools that focus on a paperless system, saving time and resources. With your migration away from paper and use of increased automation, you drastically reduce risk of manual error, which results in overall cost savings.
Start Saving Today
Deliver Safer Products Faster
Greenlight Guru ensures your processes align with leadership’s go-to-market timelines. Automation allows you to integrate quality and risk management into your design and development processes from the start — ensuring acceleration of your overall timeline.
Explore Product Development
Gain new visibility and mitigate risk
Incorporate risk controls into the product development process with the only cloud-based risk management solution that aligns with ISO 14971:2019. Demonstrate a risk-based approach to design with full traceability to related Design Controls and Components.
Learn More About Risk
Automatically maintain full traceability
Easily manage multi-level device requirements in your traceability matrix and generate a completed Design History File on demand. Create detailed design control objects, link complex configurations, and attach documents with a single click.
Streamline Traceability
Leverage educational content from product development experts
With Greenlight Guru Academy, you’ll learn practical lessons and be able to access comprehensive certifications applicable to the entire MedTech industry.
Start Learning TodayBuilt for Customers Like You

Greenlight Guru has differentiated us from other start-ups. Our auditors told us, "Wow, you're steps ahead of anyone else."

Greenlight Guru has differentiated us from other start-ups. Our auditors told us, "Wow, you're steps ahead of anyone else."



















