Streamline Production and Improve Quality Processes
Discover the only out-of-the-box solution designed to help IVD companies navigate the medical device regulatory environment and get products to market faster, with less risk. Simplify regulatory compliance and provide a single source of truth by connecting the management of all product development, regulatory, and quality processes from documentation and design controls to submission and ongoing compliance.
 Calculate Your ROI
Calculate Your ROI
Don’t Reinvent the Wheel
IVD devices are different from medical devices and are, therefore, treated differently. But they’re still subject to the same pre- and post-market controls. In terms of industry best practices and solutions for ensuring the delivery of high-quality devices, rely on our trusted platform to achieve efficiency and effectiveness.
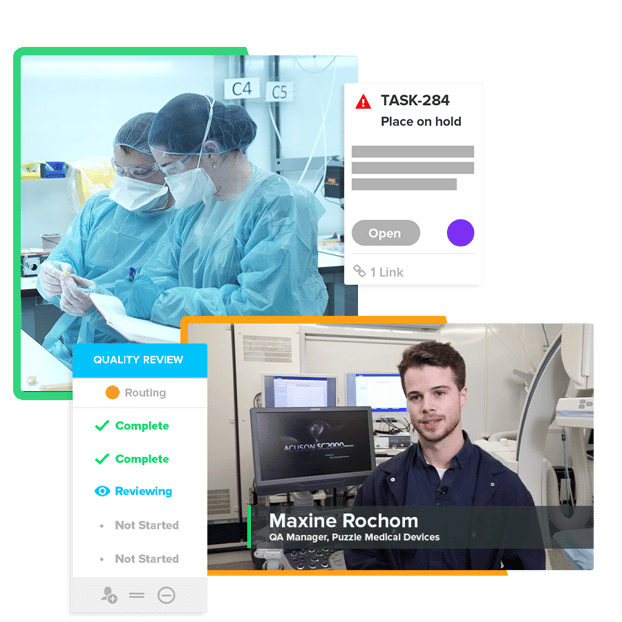
A Connected Ecosystem That Unites Your Entire Team
Pull together the power of your teams with our end-to-end solution to manage product development and quality processes. Watch your devices come to life and have confidence in the work you’ve invested in them.
Streamlined Product Management
From reagents to instruments to systems intended for use, document your design controls, build your hierarchies of components and subcomponents, and manage your product families. Streamline and automate the management of reports, matrices, and other documentation needed to develop your product and ensure regulatory compliance.
Reduce Risk and Prove Effectiveness
Identify, mitigate, and prevent potential risks early and often. Integrate risk management beyond your design. Proactively find and fix issues that expose you to risk throughout the product lifecycle.
Manage Expectations. Comply with Regulations.
An IVD is subject to the same pre- and post-market controls as a medical device. It requires a quality system and similar risk-based classification. With so many changes to the new, stringent regulations of IVDR, it’s essential to understand how to be compliant and have the solutions to do so. Let us help you simplify compliance so you can focus on building high-quality products.
Proactive PMS Planning
Simplify and stay on top of all post market quality activities and processes for monitoring and measurement of output, data analysis, and product improvement. Maintain a holistic view of the lifecycle of your product throughout all areas of your system.
A Single Source of Truth
Enhance team access to cross functional data and drive accountability by managing device requirements and quality efforts in a centralized workspace. Keep your teams aligned with the latest information, facilitate team communication, and streamline approvals using flexible review workflows with both internal and external stakeholders.
Streamlined Product Management
From reagents to instruments to systems intended for use, document your design controls, build your hierarchies of components and subcomponents, and manage your product families. Streamline and automate the management of reports, matrices, and other documentation needed to develop your product and ensure regulatory compliance.
Reduce Risk and Prove Effectiveness
Identify, mitigate, and prevent potential risks early and often. Integrate risk management beyond your design. Proactively find and fix issues that expose you to risk throughout the product lifecycle.
Manage Expectations. Comply with Regulations.
An IVD is subject to the same pre- and post-market controls as a medical device. It requires a quality system and similar risk-based classification. With so many changes to the new, stringent regulations of IVDR, it’s essential to understand how to be compliant and have the solutions to do so. Let us help you simplify compliance so you can focus on building high-quality products.
Proactive PMS Planning
Simplify and stay on top of all post market quality activities and processes for monitoring and measurement of output, data analysis, and product improvement. Maintain a holistic view of the lifecycle of your product throughout all areas of your system.
A Single Source of Truth
Enhance team access to cross functional data and drive accountability by managing device requirements and quality efforts in a centralized workspace. Keep your teams aligned with the latest information, facilitate team communication, and streamline approvals using flexible review workflows with both internal and external stakeholders.














