How to Use Queries for Monitoring Medical Device Studies in Greenlight Guru Clinical
.png?width=800&height=400&name=How%20to%20Use%20Queries%20for%20Monitoring%20Medical%20Device%20Studies%20in%20Greenlight%20Guru%20Clinical%20(new).png)
It can be said that a successful medical device clinical study is defined by its results. To achieve the best possible outcome it is necessary to generate quality data.
As Electronic Data Capture (EDC) becomes the new standard, the focus is on these systems to have built-in features that make the entire data management process easier. One such built-in feature and the focus of this blog post is the importance of queries for eCRF data. This post highlights the query functionality, how it works, and what the benefits are of using it.
What is a query?
Prior to the adoption of EDC systems, queries were collected using paper. Queries were raised manually using spreadsheets which were extremely time-consuming and not to mention the complication of accidental data being entered or removed. Furthermore, the spreadsheets could be accessed by one person at a time, complicating the whole process. In several cases, this erroneous data led to altered analysis.
Now, the use of EDC is much more common and these types of data entry errors can be dealt with at the source through Queries.
According to the CDISC a query is, “A request for clarification on a data item collected for a clinical trial; specifically, a request from a sponsor or sponsor’s representative to an investigator to resolve an error or inconsistency discovered during data review”. Subsequently the management of a query is an, “Ongoing process of data review, discrepancy generation, and resolving errors and inconsistencies that arise in the entry and transcription of clinical trial data”.
Why should you use queries in your studies?
Highlighted below are some of the reasons why you should consider using queries in clinical studies.
-
With an EDC system, Query management can be done continuously with up-to-date data because the system is continuously updated which saves time and resources.
-
In the case of incorrect data, electronic queries are generated automatically on values outside the user-defined range and can be answered immediately by the investigator.
-
As opposed to paper, all users with the right permissions have access to the queries generated and can respond quickly.
-
An example that highlights the benefits of queries is that the data can be monitored remotely, and check for instance: if the medication registered at a later stage might be in conflict with the inclusion/exclusion criteria and then consider whether the subject should be excluded from the study, or they remain included.
-
Furthermore, fully validated EDC systems document all corrections made to the data which allows the users to keep track of the status of the query.
How do queries work in Greenlight Guru Clinical?
The way queries work in Greenlight Guru Clinical (formerly SMART-TRIAL) is that if data entry staff have entered data that is inaccurate a certain flag or ‘QUERY’ is raised. In Greenlight Guru Clinical there are two types of Queries:
-
Auto/system-generated
-
Manual/human-created
The first type of query is ‘system-generated’ which acts as a flag that is automatically generated when the data entered falls outside the predefined values. This type of query needs to be marked as ‘closed’ for it to be resolved and is added into the Query list, accessed via the left-side menu in Greenlight Guru Clinical.
The second type of query is ‘manually-created’ and starts when an investigator or monitor finds inaccurate or missing data. Similar to system-generated queries, these queries need to be closed for it to be resolved.
How to use queries in Greenlight Guru Clinical?
In Greenlight Guru Clinical queries can be raised in the form of comments or a message on specific eCRF data points. All studies in Greenlight Guru Clinical have access to queries, but only users with the ‘Query permission’ will be able to create them. These users receive email in-app notifications when any query related activity takes place.
Creating system-generated queries in Greenlight Guru Clinical
System-generated queries in Greenlight Guru Clinical are triggered when Validation Rules are added during Form creation. This automatically compares the entered data in an eCRF against the values set in the Validation Rule.
When creating a Validation Rule you choose between different operators like ‘larger than’ or ‘smaller than or equal to’ to create checks on e.g., numerical and date- and time-based inputs. As a part of setting up the rule, you have to specify what should happen if the value is outside the range specified. Here, you can trigger that a query should be generated in Greenlight Guru Clinical which denotes it as a 'System Generated Query'.
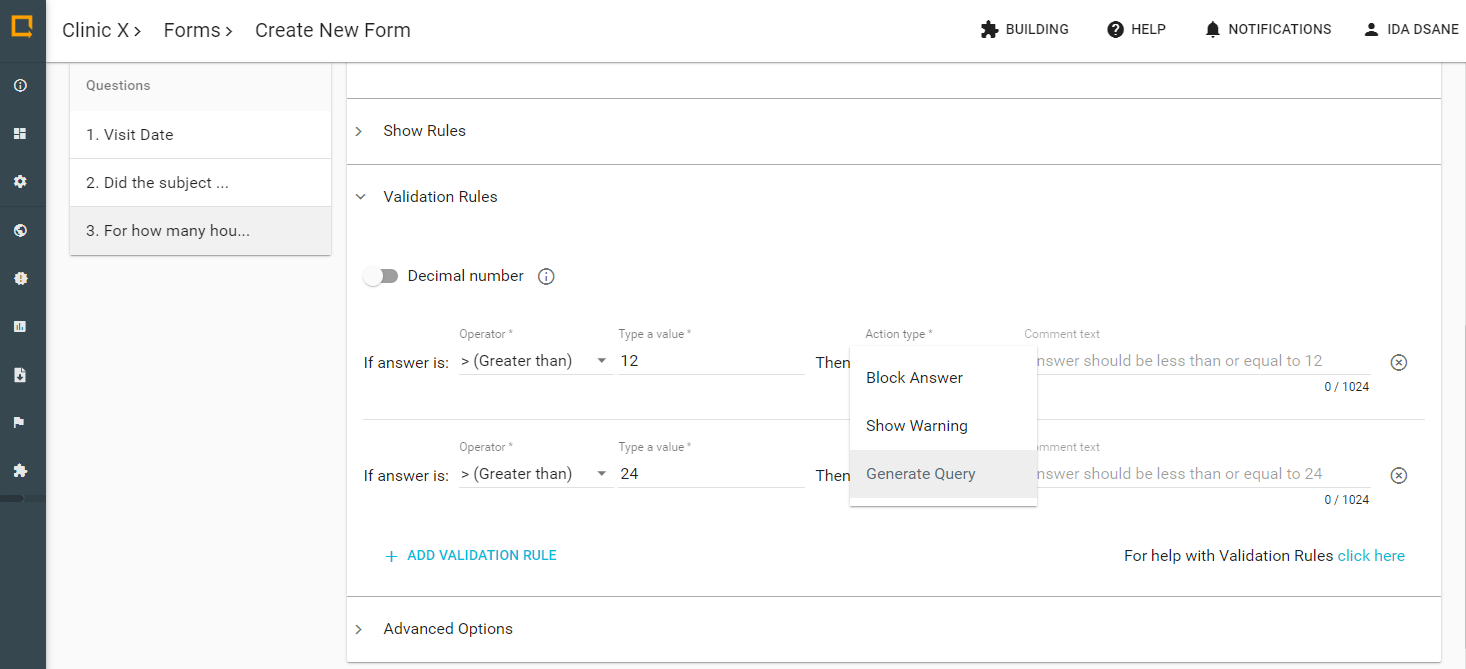
Additionally, similar to commonly known ‘edit checks’, Greenlight Guru Clinical can validate if an answer from a specific Form violates a condition which is related to another answer. For example, you can create a Process Validation Rule, which checks if an answer is ‘greater than or equal to’ another answer in a previously completed Form (or Data Event).

The data entry personnel or monitor can respond to a system generated query either with or without changing the answer of the question. They do this by reviewing the data, and navigating to the query in question. By choosing to respond to the query the user will have to specify in the dialogue box the reason for accepting and/or editing the entered data.
By setting up system generated queries you will improve data quality by minimizing confusion and eliminating input errors.
Click here to watch a short how-to video to see system generated queries in action.
Creating manual queries in Greenlight Guru Clinical
With the query permission, creating a manual query in Greenlight Guru Clinical is easy. When you as an investigator or monitor encounter inaccurate Form answers you can create a query. On choosing to create a query a dialogue box will appear where you are to ask a question or make a comment.
Once you have added in your comment the query will appear underneath the question with the name of the person creating the query, date, and time-stamp of query creation. The query will be added to the Query list and study personnel will get notified that a query has been created.
Responding to the query requires data entry personnel to either respond with or without changing the answer of the question. In either case you need to fill in a comment based on your activity. The monitor can then accept or decline the response. If the monitor declines the comment, the site personnel will have to respond to the query again, until the monitor accepts the data entry personnel’s response.
This process continues until the involved study personnel come to an agreement. On coming to an agreement and the monitor accepts the response made by the data entry personnel, the query will be closed. Queries need to be marked as 'Closed' for them to be completed. All actions are documented and accessible from the Audit log and query list view.

Both types of queries are available in the 'queries list' which is accessed via the left-side menu in Greenlight Guru Clinical. This gives you control over the queries and provides you with an outline of their status as shown in the image below.
Click here to watch a short how-to video on manually generating queries in Greenlight Guru Clinical.
Final thoughts
Clinical study outcomes are very much dependent on the collection of accurate quality data. However, we are all prone to make mistakes, it can happen! But if there are several inconsistencies in the data collected this can impact the entire study negatively. To ensure that you are collecting accurate and compliant data there are several procedures that you can implement and Queries is one such proven way.
Other important features that can help you in your clinical data management are AE/SAE reporting and Randomization.
Jón Ingi Bergsteinsson, M.Sc. in Biomedical Engineering, is the co-founder of Greenlight Guru Clinical (formerly SMART-TRIAL). He was also the technical founder of Greenlight Guru Clinical where he paved the way for the platform’s quality standards, data security, and compliance.










