Quality Management Consulting: How and When to Seek Help

A quality management system (QMS) is at the heart of every successful MedTech company. A QMS comprises all the policies, processes, procedures that ensure the production of safe and effective medical devices—which means that problems with your QMS can quickly become problems with your products.
That’s why many MedTech companies decide to seek out quality management consulting. They may need help implementing their QMS, it may be time to overhaul processes that just aren’t working as they should, or they may want to make things work that much smoother. Whatever the case, a quality management consultant can be a valuable resource for companies of any size that are looking to improve their processes, reduce wasted time, and build a culture of quality - not just compliance.
If you’re thinking about hiring a QMS consultant, here’s what you’ll want to consider as you go through the process.
BONUS RESOURCE: Click here to download the 4 cornerstones of an effective eQMS for free!
How to tell if you need a quality management consultant
Sometimes MedTech companies will proactively turn to quality management consulting when they realize they don’t have the internal resources to implement, manage, or improve their QMS. But there are also some telltale signs that your organization may benefit from a QMS consultant. These can include:
-
Frequent quality issues or product recalls. Every MedTech company will deal with nonconformances, CAPAs and other issues related to their quality system. But if these quality events are becoming routine, slowing down work and taking up valuable resources, it may be a sign that you could use quality management consulting services.
-
Inefficient or outdated processes. If you’re listening to your team and getting internal feedback that certain processes are not working—or procedures are not being followed as they should—that’s a sign that you may need to overhaul parts of your QMS.
-
Upcoming audits or certifications. If you’re not confident in your QMS, it’s a good idea to get an outside perspective on your quality system prior to attempting certification for a standard like ISO 13485. And it never hurts to get a second opinion if you’re worried about what your next audit may find.
What do quality management consulting services actually provide?
Quality management consulting covers a fairly wide range of services, and what you need from a consultant will be based on your unique circumstances. However, some of the most common services that a QMS consultant will provide include:
-
Designing and implementing a QMS. If you’re a small company, just starting your journey to market, you may not have the resources to hire full-time employees dedicated solely to your QMS. That’s where a quality management consultant can help, getting you up and running with the must-have processes for design and development, and helping your QMS grow as you make progress and need to add new processes.
-
Assessing your current QMS. This service may be particularly helpful if you already have a QMS, but are having trouble with completing change orders, closing CAPAs, document control, or other problem areas - whether you have a product on the market or not. A consultant can analyze your current QMS and make recommendations for improvements in your processes and workflows to ensure you go beyond the bare minimum of compliance and begin building a culture of quality.
-
Quality training. Training management is one area of your QMS that you can always count on auditors reviewing. So it’s essential that all employees are trained on the right processes and that their training is formally documented—including proof of its effectiveness. That can be difficult even for mature MedTech companies, which is why many organizations use quality management consultants to help implement their training program.
-
Internal audit support. You can think of your internal audits as the dress rehearsal for audits by FDA or notified bodies. Internal audits are meant to catch any potential problems and solve them before an external auditor comes to review your QMS. Many consultants use Certified Auditors to conduct internal audits for their clients, ensuring the same level of rigor that you’ll undergo during an external audit.
-
International regulatory support. Medical device regulations vary across different countries and regions, and having a QMS that conforms specifically to US regulations, for instance, does not mean you’ll automatically be compliant with regulations in other parts of the world. If you’re looking to expand to new markets, a quality management consultant can help you develop a regulatory strategy to ensure a smooth market entry.
How to find and source a reliable quality management consultant
If you’re ready to start looking for quality management consulting services, you will need to do your due diligence up front. This is an incredibly important partnership, and a good QMS consultant can change the trajectory of your business.
You’ll want to evaluate consultants as you’d evaluate any change to your QMS. Start by identifying and clearly articulating your organization’s needs, as well as all the stakeholders that should be involved. In a typical MedTech company, this will include Product Development, Regulatory Affairs, Quality, Manufacturing, and Executive Management.
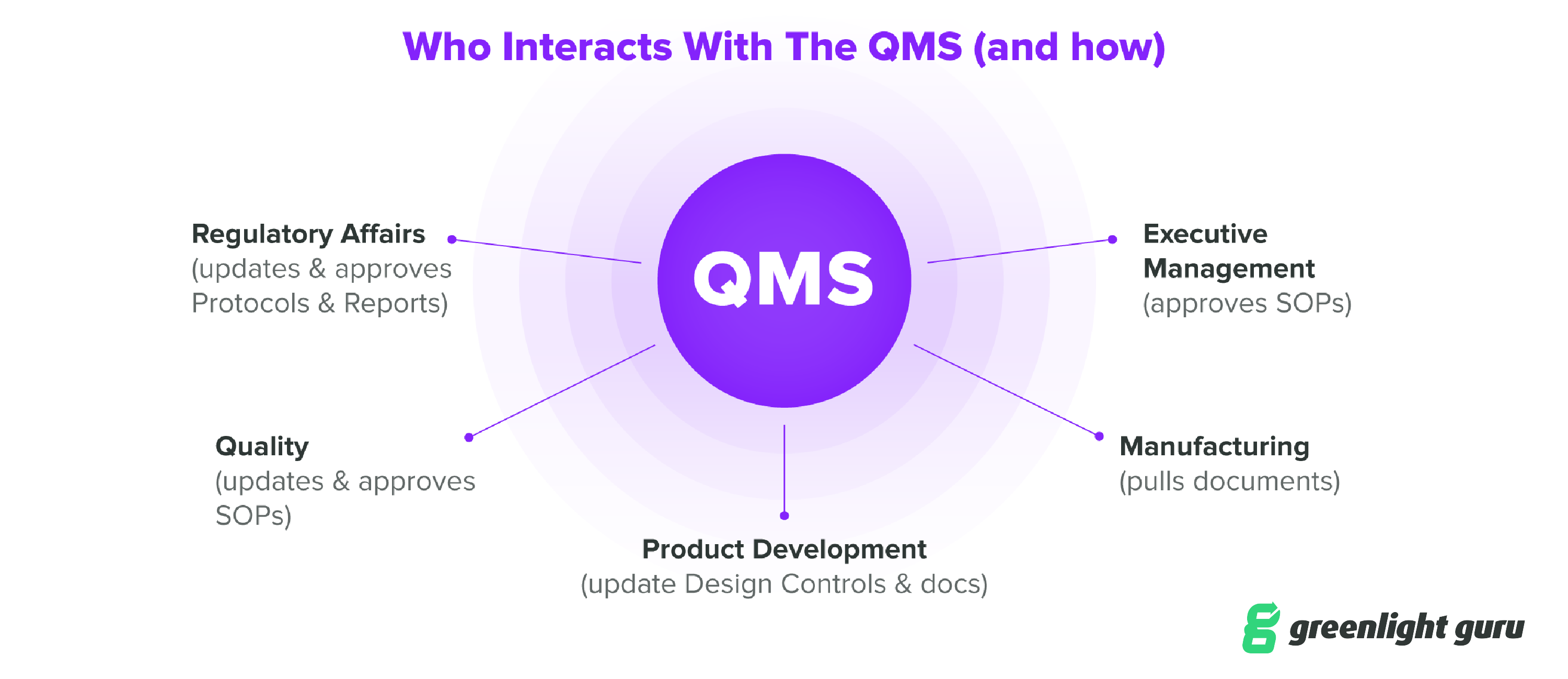
You’ll want to evaluate at least a few different consultants to get a sense of the services they provide and their costs. It’s also a good idea to get references from former or current customers to help you get a better sense of how each potential consultant works with their clients.
If you’re unsure where to start looking, consider asking other MedTech companies or suppliers of other MedTech companies. For instance, at Greenlight Guru, we’ve developed relationships with consultants and service providers throughout the MedTech industry, including some of the most experienced quality management consultants. All Greenlight Guru customers have access to our Partner Ecosystem, where you can search through a deep roster of potential partners to find the one that’s right for you.
BONUS RESOURCE: Click here to download the 4 cornerstones of an effective eQMS for free!
Get a QMS solution built for MedTech with Greenlight Guru
An excellent quality management consultant can do wonders for your QMS, but the problems we’ve discussed in this article often indicate deeper issues with the way a company is managing its quality system. If you’re struggling with inefficient processes and frequent quality issues—or you aren’t certain your QMS is audit-ready—it may be time to look for a new QMS solution.
At Greenlight Guru, we built our QMS software specifically for MedTech companies that need a modern, compliant, and flexible solution for their quality system. With end-to-end traceability, automations for quality events, and built-in compliance with FDA and EU regulations, you’ll have everything you need to feel confident in your QMS again.
You’ll also have the industry’s top-rated customer success team on hand to help guide and assist you from day one. And if you’d still like some outside help from a quality management consultant, you’ll always have access to our exclusive Partner Ecosystem, which makes choosing the right consultant simple.
Implementing and maintaining a highly effective and compliant QMS has never been easier. Get your free demo of Greenlight Guru today→
Etienne Nichols is the Head of Industry Insights & Education at Greenlight Guru. As a Mechanical Engineer and Medical Device Guru, he specializes in simplifying complex ideas, teaching system integration, and connecting industry leaders. While hosting the Global Medical Device Podcast, Etienne has led over 200...
Related Posts
eQMS Buyer’s Guide: Making the Business Case for a New QMS Solution
Top 50 Medical Device QA/RA Consulting Firms
Best QMS Software: Ultimate Guide to Comparing Quality Management System Solutions
Get your free download
4 Cornerstones of an Effective eQMS










