Quality Assurance vs. Quality Control in the Medical Device Industry

Imagine that your medical device malfunctioned during patient use. Do you know whether quality assurance or quality control is responsible? When working through remediation efforts, do you know which quality function demands the attention, or should you make improvements to both?
More often than we may like to admit, a vast majority of medical device professionals do not have a full grasp on the acronyms and meanings of core industry terminology relevant to their role.
Quality assurance and quality control are complementary parts of a quality management system, yet serve very distinct roles with different purposes. Without knowing how the two quality functions differ and intersect, your QMS processes will struggle, and more importantly, you'll struggle to ensure your medical device is safe and effective.
This article will clear up any lingering confusion around the two terms quality control and quality assurance, explain how they differ from one another and how they intersect, and how professionals in these roles can leverage certain tools and methodologies to successfully control quality and assure quality.
Difference between Quality Assurance & Quality Control
What is the main difference between quality assurance and quality control? Quality assurance is proactive and process-focused and proactive in nature. Quality control is product-focused and reactive in nature. The combination of the two is what gives your medical device company the greatest likelihood of attaining the highest level of quality.
-
Quality assurance prevents flaws in the way a medical device is manufactured. Quality assurance happens throughout the medical device manufacturing process. Those responsible for quality assurance look for problems in processes that might result in nonconforming products, and fix those processes that would otherwise cause defects.
-
Quality control finds flaws in products after they’ve been manufactured but prior to distribution into the marketplace. Quality control tests products or batches of products to see whether they conform to product specifications or if nonconformities exist. The goal is to catch defective products before they’re shipped to the end user.
Let's take a closer look at each to better understand the unique distinctions between quality assurance and quality control.
What is Quality Assurance (QA)?
The primary focus of quality assurance is the process.
Processes can be healthy or unhealthy, effective or ineffective, efficient or inefficient. It’s the job of quality assurance to maintain and improve those processes such that your company can better ensure final products that are high-quality.
Quality assurance isn’t a single step, method, or tactic. Quality assurance involves many different methods performed ongoing for flaw prevention. The end goal is continuous process improvement of product development and quality management, such that any flaws are reduced significantly if not completely.
What is Quality Control (QC)?
The primary focus of quality control is the product.
Despite product teams best efforts, supported by quality assurance helping hew to good processes, some flaws manage to slip by undetected. When the product is finally complete, it’s time for quality control to step in.
Quality control is the final line in the medical device manufacturing process. Quality control checks whether the soon-to-be delivered product is actually free of flaws. If it is, then you can be more confident in its success. If it’s not, you can feel relief knowing your quality control team found a defect before a patient experienced one.
Quality Assurance vs. Quality Control Roles in the Medical Device Industry
Quality assurance and quality control professionals share the same goal—ensuring the production, manufacture and delivery of high-quality products and services—but assume different roles within a medical device company.
On average, a typical medical device company will have multiple people dedicated to quality assurance and at least one person dedicated to quality control.
Role of Quality Assurance
Quality assurance staff oversee the medical device manufacturing process to ensure that it meets external standards from regulatory bodies, such as FDA and ISO, as well as internal standards from your company.
Staff in charge of quality assurance testing generally stick to a formula called the Deming Cycle—also known as the PDSA Cycle. The figure shown below depicts a four-step process that summarizes the primary quality assurance responsibilities.
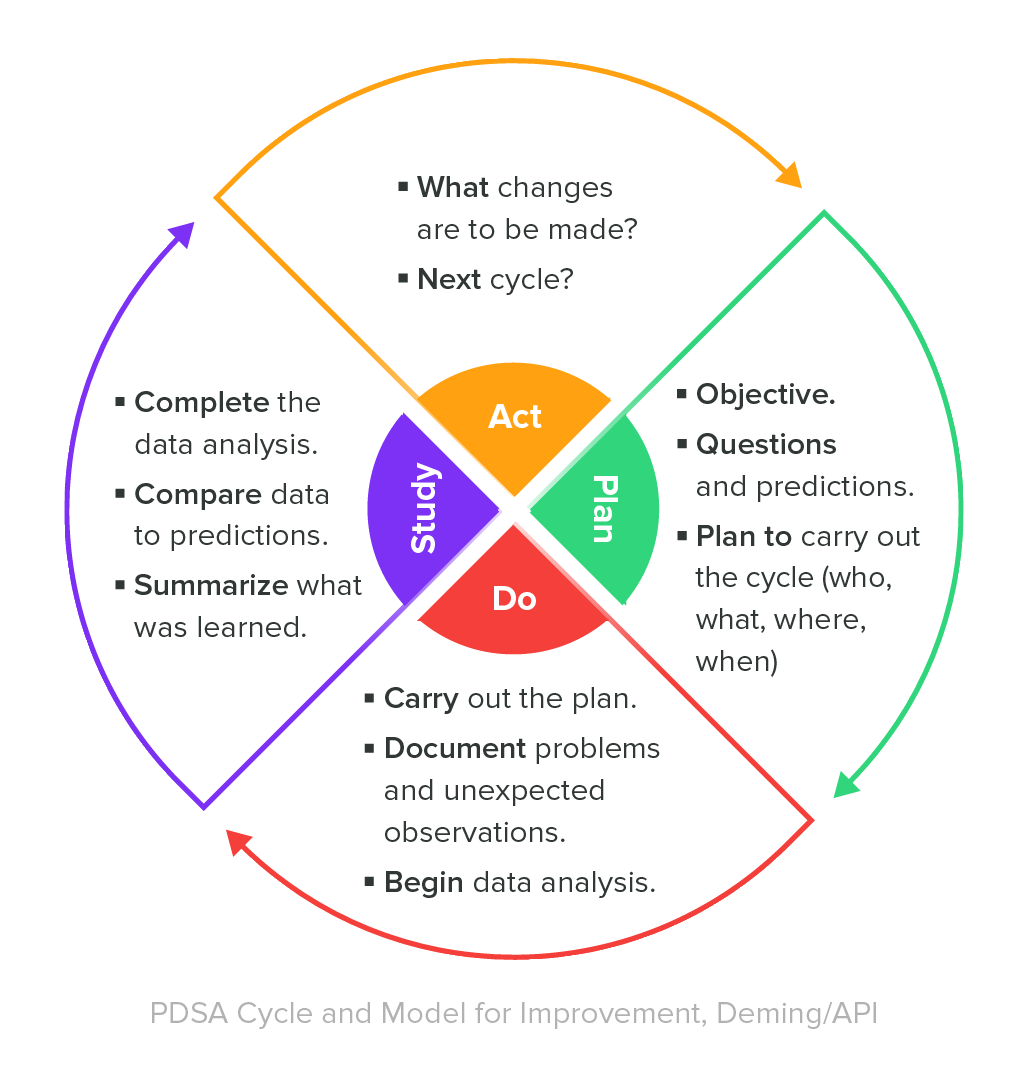
Combined with the PDSA Cycle, quality assurance teams use a number of tactics to offer continuous process improvements. The primary methodology used by QA staff during this process is called quality assurance testing and it highlights the reason why we refer to quality assurance as process-oriented.
Quality assurance testing monitors the medical device manufacturing process to determine whether all requirements are being met. The goal is to identify and correct product flaws, eventually leading to process improvement and an overall decrease in defects.
There are four common QA testing tactics that quality assurance professionals use:
-
Quality audits: where quality staff inspects internal manufacturing procedures and verify whether regulatory requirements are being met. These types of audits typically take place at predetermined times.
-
Process definitions: where quality staff determines exactly what a process looks like and how it works. Clarity here is essential to conducting testing processes that deliver consistent results.
-
Tool identification and selection: when quality staff selects new equipment or tests existing equipment. For instance, quality staff might test whether a particular manufacturing tool is reliable and whether it can consistently create parts that meet requirements.
-
Staff training: how quality staff ensure staff across the organization are adequately prepared to handle the processes they’re in charge of. Quality staff can recommend more training if nonconformities indicate it’s necessary.
The PDSA Cycle runs continuously, giving quality assurance staff the chance to renew their objectives and implement improved ways of meeting those renewed objectives.
Role of Quality Control
Unlike the assurances for continuous process improvement described above, quality control begins after product development is complete. The primary concern of someone in the quality control role is the product, rather than the processes that generated the product.
Quality control teams have a variety of tools at their disposal to fulfill their job duties. Generally, these methods involve either testing or inspecting the manufactured products to determine whether they conform with predefined product requirements or to find and isolate nonconformities.
Product testing doesn’t begin until a batch or lot of medical devices is ready for shipment. Once a product reaches this point, quality control staff are responsible for inspecting and giving final review of the batch or lot to ensure that the devices therein are, in fact, ready for sale.
There are three primary tactics used by quality control teams to test and inspect products for conformity or nonconformity:
-
Acceptance criteria: quality control staff are required by FDA to establish a formal document detailing how they will determine whether products conform to product specifications. Quality control staff are responsible for documenting said acceptance and tracking which products or batches met this criteria and which did not.
-
Product testing: how quality control staff inspect, test, and verify whether a product is ready for shipping. Under FDA’s quality system regulation, medical device manufacturers have the freedom to design their own quality control tests, but must provide detailed documentation that proves the efficacy of this verification.
-
Corrective and preventive action (CAPA): These investigative events are triggered when quality control staff find a nonconforming product, perform a root-cause analysis, and discover a systemic flaw that led to the nonconformance.
CAPA is the most important tool quality control staff have. With this being the case, companies mustn't overuse this tool. A common mistake made by quality control teams is being too quick to initiate a CAPA investigation for any and every issue found. Remember, while CAPA is an effective tool, it’s best suited for defects that reveal potential systemic flaws.
Managing Quality Assurance & Quality Control Activities in a Quality System
QC (quality control), QA (quality assurance), and QMS (quality management system): three 'Q' acronyms for quality-related terms that are easy to mix up. Part of the confusion has to do with how and where they intersect with one another, which can be easily cleared up by keeping a few simple things in mind.
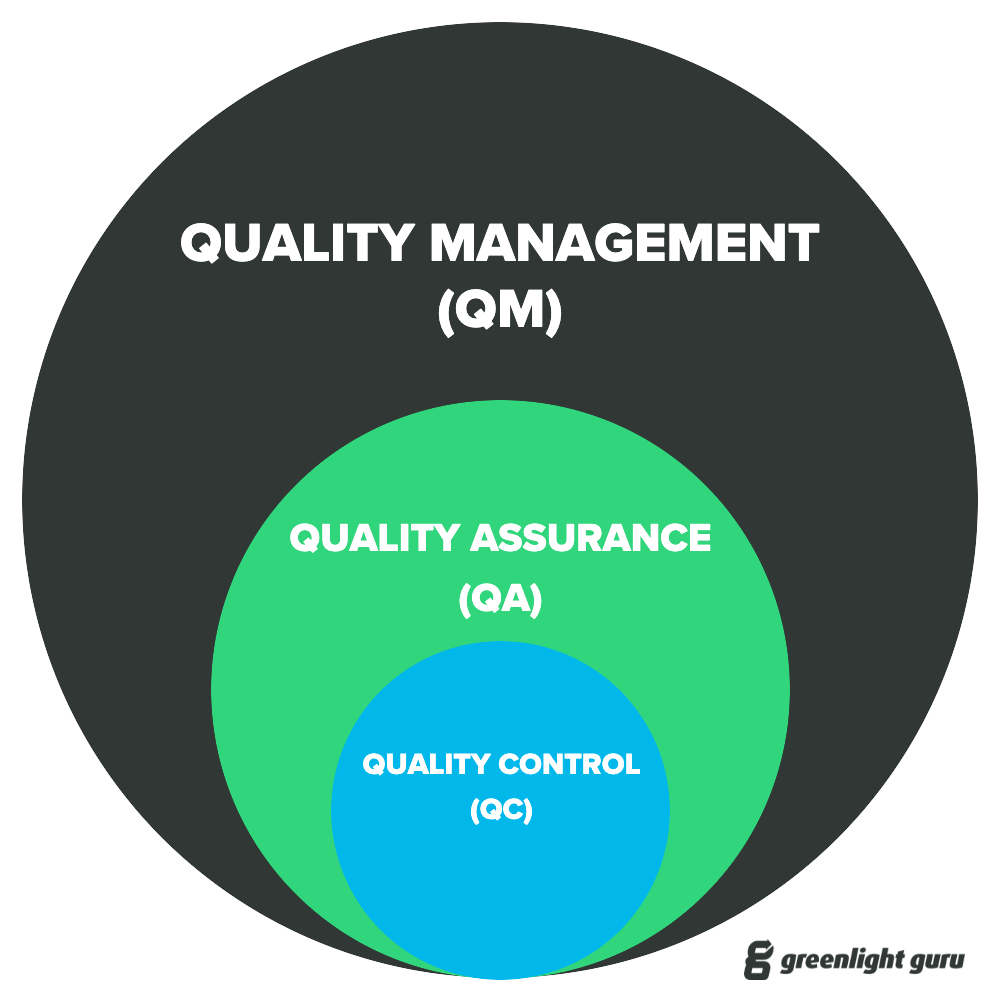
Both QA and QC activities are carried out through various quality management processes. Quality assurance teams will use a quality management system as one of their tools, for instance, to assure quality; however, QMS processes involve more than just the functions of quality assurance and quality control.
A quality management system supports many functions—assuring and controlling quality being among them—but its overarching goal is to manage quality at the planning and execution stages. Quality management systems formalize all policies, procedures, processes, and other data that companies use to ensure the creation of safe and effective products.
For medical device companies, a QMS is the engine that powers pre- and post-market subsystem processes, like design control, risk management, and CAPA. It is also where key company artifacts live which are used to make a device, like a quality manual, design specifications, and standard operating procedures (SOPs).
It is the QMS documentation that dictates the various quality assurance and quality control functions carried out by a company and serves as proof that such activities took place as necessary for compliance purposes.
Achieve the Best Quality Outcomes with a Medical Device QMS Solution
For medical device manufacturers that take quality assurance and quality control seriously, leveraging a purpose-built QMS solution is essential.
Here are just a few ways you can achieve greater quality outcomes with a medical device-specific QMS solution like Greenlight Guru:
-
Demonstrate closed loop quality system traceability. With a QMS built for medical devices, quality assurance staff can easily and immediately access product specification data, using it to ensure a product meets every necessary requirement.
-
Document, design, purchasing, production, and supplier controls built into the system according to the latest industry best practices. Greenlight Guru's software makes it easy for quality assurance teams to oversee these processes and ensure everyone is following the most up-to-date processes.
-
Conduct internal audits with ease. Greenlight Guru has a dedicated Audit Management workflow built into the system that enables teams to proactively catch quality issues before they reach the quality control stage.
-
Automated quality event management. Greenlight Guru's QMS software streamlines the quality event process by connecting all related quality system data for faster root cause determination.
Greenlight Guru’s QMS software is the only quality management solution made by medical device professionals for medical device professionals, providing a best-in-class solution for all quality assurance and quality control needs. Get your free personalized demo of Greenlight Guru today!
Looking for an all-in-one QMS solution to advance the success of your in-market devices that can integrate your post-market activities with product development efforts? Click here to take a quick tour of Greenlight Guru's Medical Device QMS software →
Jon Speer is a medical device expert with over 20 years of industry experience. Jon knows the best medical device companies in the world use quality as an accelerator. That's why he created Greenlight Guru to help companies move beyond compliance to True Quality.
Related Posts
How to Determine the Operational Quality of a Quality System Using a Performance Assessment
5 Myths Engineers Believe About Quality (and what is really true)
FDA Case for Quality: 2018 Comprehensive Review
Get your free resources
3 Free Templates, Nonconformance, CAPA, and Complaint Management










