Quality assurance testing is the aspect of quality management that focuses on providing confidence that quality requirements will be fulfilled through proactively isolating and correcting sources of manufacturing defects.
Medical device companies that wish to sell their devices in the United States and in markets around the world must implement a quality system that is compliant with regulatory standards to ensure devices that are manufactured and sold are safe and effective for the intended user.
The goal of quality assurance testing is to effectively monitor the medical device design and manufacturing process, ensuring that the requirements for the medical device are fulfilled. Quality assurance testing activities focus on the improvement of the processes that generate finished medical devices, with the objective of reducing the overall occurrence of product defects.
Quality Assurance and the PDCA Cycle
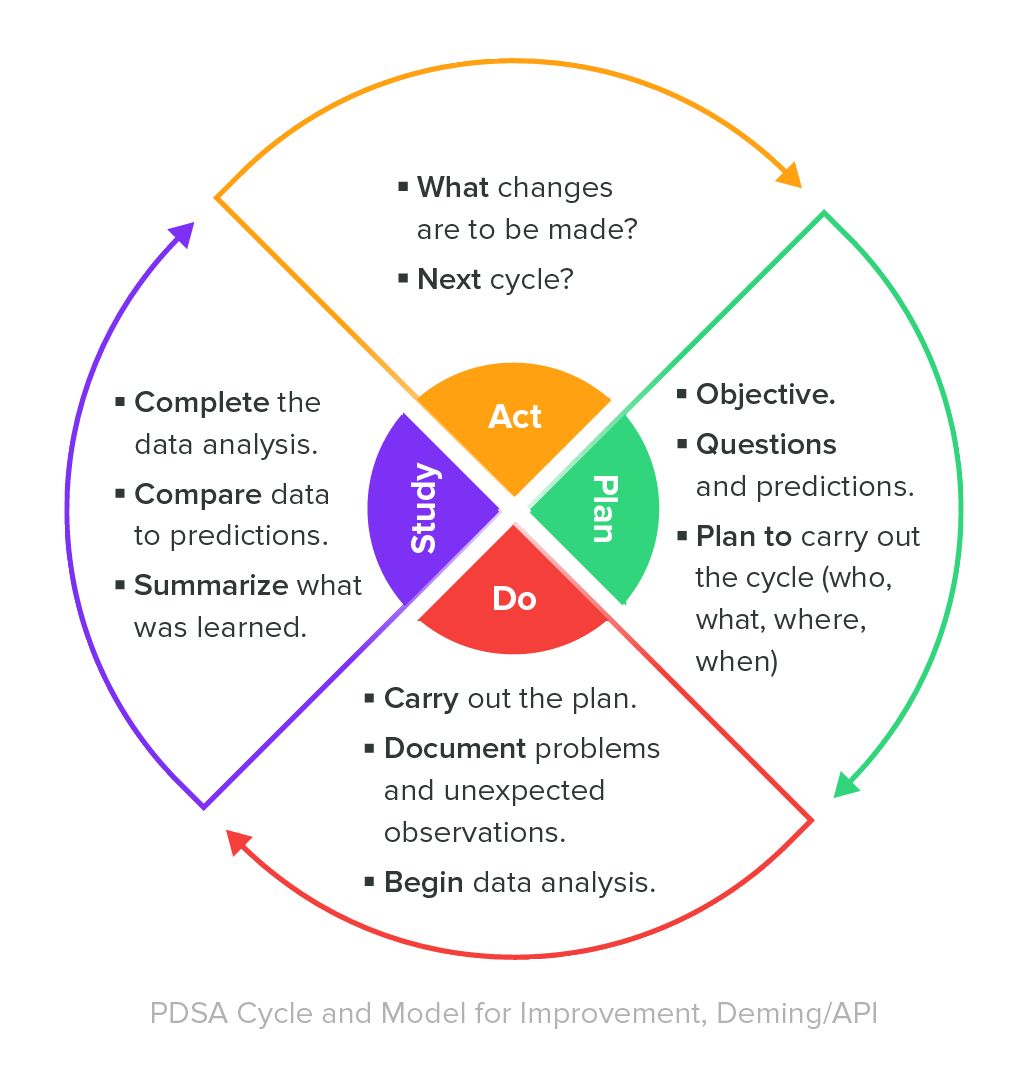
Traditional quality assurance testing practices were based on the Deming cycle, also known as the PDCA cycle for its four components: Plan, Do, Check, Act.
Plan - Medical device companies should establish quality objectives for each stage in the manufacturing process, and determine how to plan or design processes to meet those objectives.
Do - In this stage, medical device companies implement their planned manufacturing processes. Process changes can also be implemented as part of the "Do" stage.
Check - Once manufacturing processes have been designed and implemented, medical device companies must continually monitor these processes, check whether they adequately meet the quality objectives defined in the “Plan” stage, and modify processes when required.
Act - In the final stage of the Deming cycle, medical device companies implement any process improvements that are necessary to improve the quality of produced medical devices and meet the requirements or quality objectives of the “Plan” stage.
Quality Assurance (QA) vs Quality Control (QC) - What's the Difference?
Quality assurance (QA) and quality control (QC) are sometimes used interchangeably to describe processes that ensure the quality of manufactured medical devices. However, quality assurance and quality control are separate processes that occur at different times with respect to the manufacturing process and the product life cycle.
Quality assurance testing is a proactive, process-oriented initiative that seeks to identify and rectify process issues that can lead to nonconformances. Quality assurance testing may be conducted by a quality manager or other quality-focused staff member, but the ultimate goal is preventative - to reduce the number of manufacturing nonconformances that occur.
In contrast, the quality control aspect of quality management takes place once products have already been manufactured. Quality control focuses on the products themselves, not the manufacturing process. The goal of QC is to ensure that nonconforming products manufactured by the company are not sold to consumers. Quality control activities include testing and inspections of manufactured goods to ensure their conformity with device quality requirements.
When a quality control inspection uncovers instances of nonconforming products, the medical device company can initiate a corrective and preventive
Quality Assurance Testing Activities for Medical Device Companies
Quality Audits - Internal quality audits help medical device companies ensure their compliance with internal manufacturing procedures and regulatory requirements. Quality audits also formalize the monitoring of procedures and products at predetermined intervals. Internal quality audits can trigger CAPA activities when it is discovered that any aspect of the manufacturing process is not performing adequately.
Process Definition - Processes are designed to produce predictable results over time, which directly translates into quality products that meet the desired specifications. Clear and accurate process definitions are a quality assurance activity that proactively
Tool Identification and Selection - Selecting the right tools and equipment for use in the manufacturing process is a necessary step for ensuring that manufactured products meet the intended product specifications. Quality assurance testing can include specific tests that determine whether a chosen tool can be used reliably to build products with the desired specifications. If the implemented tool proves to be inadequate, it should be replaced by a different one that can accomplish the intended purpose.
Staff Training - Staff
Greenlight Guru's eQMS Software Supports Quality Assurance Testing
Medical device companies must maintain complete records of their quality assurance testing activities, including detailed process definitions and internal audit records. Greenlight Guru's eQMS software is specifically designed for medical device companies and provides its users with a single source of truth for quality documentation. The cloud-based platform serves as a secure repository for QA and QC activities and records, streamlining the quality management process for companies to manufacture and sell safe, true quality medical devices.
Related Links
Looking for an all-in-one QMS solution to advance the success of your in-market devices and integrates your quality processes with product development efforts? Click here to take a quick tour of Greenlight Guru's Medical Device QMS software →








