How to Escape the Lure of Legacy Quality Management Systems

Many device professionals in small to medium-sized businesses (SMB) still use legacy systems to support their quality management processes.
According to data-driven insights from our survey of over 500 industry respondents of our 2020 State of Medical Device Product Development and Quality Management Report, implementing a legacy quality management system (QMS) can lead to a cascade of problems—budget strain, inefficiency, and risk management issues, to name a few.
Furthermore, the longer you use a quality system that isn’t purpose-built to manage medical devices, the more you accrue technical debt and the harder it is to improve your quality management processes as you scale.
To escape this cycle, medical device companies must confront their technical debt and rebuild their infrastructure with modern quality tools that are equipped to scale as the business grows.
FREE DOWNLOAD: Click here for the top 5 things you should focus on for the remainder of 2020.
Beguiling lure of Legacy QMS
Legacy quality management tools appear attractive to medical device companies at first.
Paper-based or "digital paper" systems seem clean and simple when your team is small and just starting out.
Tools like Google Drive to manage documents, forms, and procedural paperwork seem accessible if quality management isn’t your priority yet; legacy, general-purpose QMS solutions seem trustworthy, tried and true.
During the early stages of growing your company, asking your team to think about quality management processes can induce exasperation rather than excitement.
The prospect of implementing a QMS and setting up design controls or risk management procedures can create exhaustion before the implementation has even begun.

This is the standpoint from which many QMS software decisions are made—a legacy solution early on seems “good enough.” Let’s look at some of the major reasons medical device companies settle for it.
Short-term gain for long-term pain
Adopting a legacy QMS or leaning on general-purpose office tools may feel like an easy way to satisfy regulatory checkboxes and move through product lifecycle stages quickly.
This is especially tempting for small medical device companies. Since much of a smaller shop's viability depends on marketing their product, there's a common temptation to adopt the first system they see without doing the due diligence and proper research to validate this long-term investment.
In fact, according to our Industry Benchmark Report, the smaller the company, the more likely it’s using legacy quality management technology. Our research shows that 65% of VSBs still use legacy systems to support quality management processes.
Quality systems are rarely a day-one priority, but if you don’t prioritize quality in other ways, an effective system becomes hard to implement.
Key QMS requirements which are mandated by nearly every regulatory body, such as documenting design controls, ensuring total product lifecycle traceability, and maintaining an up-to-date design history file (DHF), become increasingly difficult to manage over time when using general-purpose systems.

Managing risks can quickly become unmanageable, your go-to-market strategy can suffer, and technical debt can build beyond repair. In many cases, adopting a legacy QMS is trading true long-term convenience for the illusion of short-term convenience.
Ad hoc risk management
Legacy QMS solutions make it difficult to be proactive about risk activities when important information becomes fractured across disparate, disconnected systems.
Without a single source of truth, it’s easy for serious risks to slip by unaddressed. If risks slip by, then risk management becomes a vicious cycle of finding new risks, updating the QMS configuration, and waiting to find another.
Since many legacy QMS solutions aren’t purpose-built for the medical device industry, not only do they require an initial configuration, but also ongoing reconfigurations.
With no purpose-built templates for procedures and no alignment with industry-specific workflows or regulations, legacy QMS solutions turn activities around managing risk into a reactive process. This makes tracking and managing risk a near impossible task.
Despite the link between risk management and the need for rigorous, technology-supported controls, more than half of the organizations from our survey say they use legacy systems to manage quality and risk management.
Of that group, 1 in 3 say risk management of their device is minimally or not at all integrated with post-market quality processes.
A single source of truth is almost impossible to establish manually. If version control is manual rather than automatic, then your team wastes time updating and tracking changes rather than managing risks.
If you can’t adequately track changes, then you may find yourself hunting down signatures or missing documents in time when it matters most, like during an audit or inspection.
Best-in-class organizations realize the benefits of integrating strong risk management activities with other key processes, such as design and post-market events like CAPA, over the course of the device lifecycle. To these industry leaders, risk is much more than merely a compliance activity.
Moves fast, but in the wrong direction
A legacy QMS or general-purpose tool may at first seem like the best way to get you to market. True quality management, you think, can come later.
The logic goes that, as a startup, you should prioritize product development and bringing your device to market. If you don’t, you won’t have a product that even warrants regulation.
The risk with that way of thinking is: the longer you use a tool that doesn’t fit your needs, the harder it is to swap it out for a better one. If the budgets are already decided, it’s difficult to redirect money toward a different tool—even if it will save money.
It’s even more difficult to weigh those long-term costs and risks against the immediate costs of buying a new tool. According to our research, 44% of businesses cite insufficient budget as a barrier to establishing best-in-class quality management. An additional 34% of businesses cited the cost of validating new tools as another barrier.
Even after you make the business case for new tools, you might find it harder than you originally thought to rebuild your quality procedures and the culture that emerges from them. Culture, despite the amorphous nature of the word, can be a real barrier. Once your organization develops a culture, it can take a lot of effort to change it.
According to our research, only 41% of organizations that say they use legacy tools agree that quality is essential to their company cultures, whereas 56% of organizations using best-in-class tools say quality is essential.

“Quality as an asset” means building quality into every stage of the business's processes and overall strategy — starting with the inception of the device all the way to when it's interfaced by end-users.
In order to achieve this, companies must adopt and embrace a culture of quality. Once it's achieved quality culture will need to be reinforced across the organization, an outcome by which is sustainable through best-in-class tools that enable it.
How to Upgrade from a Legacy QMS
Escaping the lure of a legacy QMS seems hard because many companies fall prey to the sunk cost fallacy—that it is less expensive to keep fixing what you have than to start new. This creates a negative feedback loop that’s difficult to escape.
A legacy QMS solution requires regular time and money investments, whether it’s to reconfigure the QMS or redesign your processes to fit its workflows. Those investments, despite and because of their costs, make medical companies hesitant to jettison that work and “start over.”
The only way to defeat the sunk cost fallacy is to think beyond whatever investment you’ve already made and think about the kind of company you want to be.
The best time to adopt best-in-class tools is yesterday; the second-best time is today. To do so, you’ll need to right-size your QMS, become more agile, and evaluate the QMS vendors that will support your efforts.
Right-size your QMS
Right-size your QMS and build out the processes you need, when you need them. Your QMS should reflect the size, state, and growth stage of your company.
Some of this decision-making may depend on your investors, if applicable. Many investors will ask whether you've obtained ISO 13485 certification, in which case a modernized electronic QMS (eQMS) solution should be used. If your company isn’t yet at that point, then you can likely afford to think about the precise tools you’ll use later.
Early on, it’s better to focus on the content of your procedures rather than their form. If you record the relevant content, then you can formalize it properly later. If you’re not controlling your documents, however, then you’re making problems you’ll eventually have to confront.
The key, both to building a quality culture and preparing your company for an eQMS implementation, is to use product development and product risk management best practices from the start.
As your company evolves, your quality management needs will change. Once your company has begun manufacturing, device production, or product launching and selling, your quality system should reflect that growth.
Adopt a nimble approach
Our research shows that more than seventy-five percent of companies do not consider themselves to be highly nimble and adaptable to new business operations.

When it comes to the competitive leaders in the space, agility is more commonly reported, particularly for smaller sized companies.
Larger companies tend to come with more red tape, more bureaucracy, and more stakeholders that require step-by-step approval.
The fact is that regulations apply to companies large and small, and a well-organized team can still take a nimble approach to the medical device lifecycle.
Nimble, highly competitive medical device companies are likely better able to:
- Vet and adopt new tools
- Adapt to evolving business needs
- Shift focuses to keep up with market demands
Agility can take many forms. The ability to quickly design, develop, and receive feedback on devices through rapid prototyping, for instance, enables iteration that can outpace those laggard organizations.
This high-speed evolution enables improvements with defining user needs, identifying the right materials, articulating form-fit-function requirements, and capturing the most critical human factors.
Implement tools that enable growth
Tools that are “good enough” to simply check a box or fulfill a short-term need can bottleneck your efforts in future lifecycle stages of your product as you grow.
Insights from our 2020 benchmark survey show that companies that use best-in-class tools are three times more likely to call themselves “highly efficient” when bringing a device to market.
But getting a device to market is only part of the battle.
What about the preparedness of companies who undergo an unannounced audit? Those who use best-in-class tools were also twice as likely to be “very confident” if an unannounced audit asked them to demonstrate total lifecycle traceability.
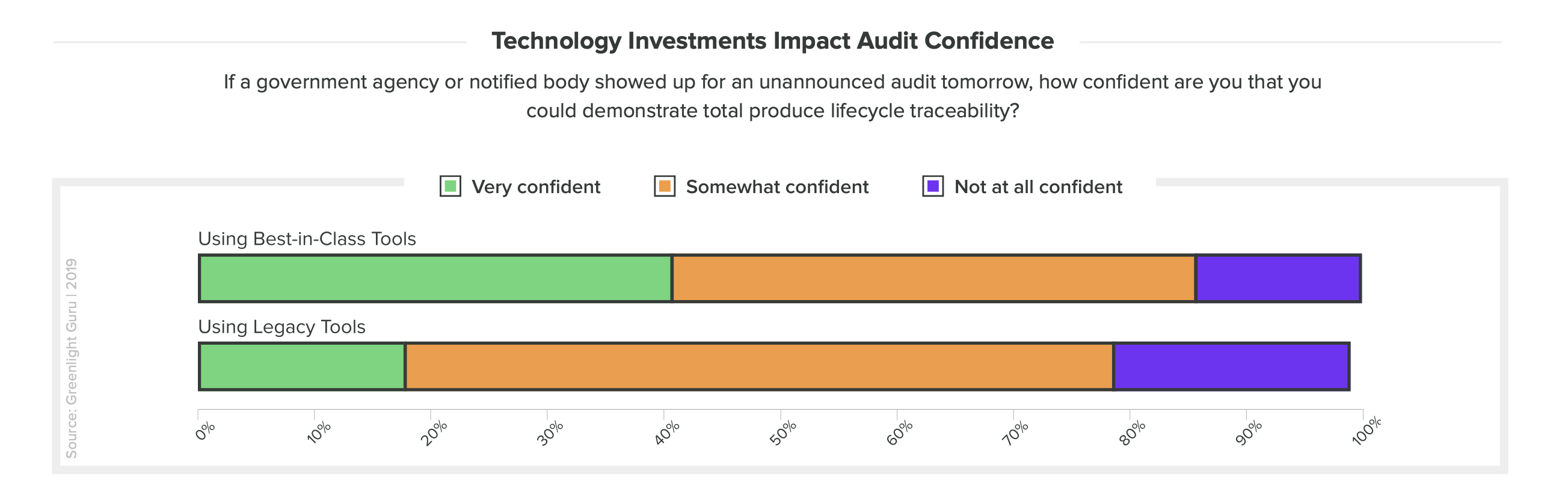
There's still a vast majority of organizations who say they use between two to five different tools to manage quality. For these companies, their quality management processes are fractured and divided. With a patchwork of technologies—some legacy, some general-purpose—these medical device companies will struggle to scale.
Implementing one of the best QMS software solutions will guide all of your efforts from start to finish, without the need of supplementary tools to fill in gaps.
Whether you’re just now thinking about setting up your QMS procedures or you’re ready to move away from your existing legacy system and adopt a modernized solution with total lifecycle traceability, Greenlight Guru can fit your product development and quality management needs.
FREE DOWNLOAD: Click here for the top 5 things you should focus on for the remainder of 2020.
Modern QMS: a Proven Alternative
Negative outcomes of risk will take fold whether you're ready or not.
The best way to manage risk, backed by the data of our benchmark report, is to invest in best-in-class tools that build quality procedures and facilitate quality culture.
The decisions you make now shape the agility with which you can make decisions later. You can—and should—wait until it’s the right time for your company to invest in a modern, purpose-built QMS.
Just remember if you bind your company to a legacy QMS solution, you might invite more risk to both your product and your business than you can manage now or eliminate later.
If your tools don’t fit the procedures necessary for medical devices, these same tools that are being used to help can end up killing your project. An investment in Greenlight Guru Quality saves you money, keeps you nimble, and sets your medical device and company up for ongoing success.
Looking for an all-in-one QMS solution to advance the success of your in-market devices that can integrate your post-market activities with product development efforts? Click here to take a quick tour of Greenlight Guru's QMS software →
Jon Speer is a medical device expert with over 20 years of industry experience. Jon knows the best medical device companies in the world use quality as an accelerator. That's why he created Greenlight Guru to help companies move beyond compliance to True Quality.
Related Posts
[VIDEO] Integrating Design Controls & Risk Management To Streamline Product Development (Make Phase)
[VIDEO] How to Prepare your QMS for a Successful Medical Device Product Launch (Release Phase)
[VIDEO] 4 Medical Device Manufacturing Best Practices for a Successful Design Transfer (Approve Phase)
Get your free PDF
Legacy vs. Best-in-Class QMS Tools Comparison Chart