How to Design a Successful Post-Market Clinical Follow-Up (PMCF) Survey
%20Survey%20(new).png?width=800&height=400&name=How%20to%20Design%20a%20Successful%20Post-Market%20Clinical%20Follow-Up%20(PMCF)%20Survey%20(new).png)
PMCF Surveys are one of the tools medical device manufacturers can use to collect data for Post-Market Clinical Follow-Up (PMCF). But a PMCF survey under the MDR is not like a traditional survey. As a sponsor, you need to ensure that the methods and data will pass the scrutiny of the Notified Bodies.
This blog will present advice and guidance on how to conduct a successful PMCF Survey, based on success stories from MedTech companies that use Greenlight Guru Clinical for that purpose.
The European Medical Device Regulation (EU MDR) is forcing many MedTech companies to redesign their clinical strategy including their PMCF plan. With new requirements on clinical evaluation, Notified Bodies can now request access to clinical data. This means that companies who want to market their medical devices in the EU must have all data that are used to support clinical claims available in-house.
After a device is placed on the market, manufacturers will have to continuously, and proactively, gather data on the device’s safety and clinical performance, which is laid out in a PMCF plan. Failing to do so, can result in a loss of CE-mark certification.
The topic of PMCF in the EU MDR is of special interest because it introduces new requirements for post-market clinical data collection. But the loose definition of a PMCF Study (in the legislative text) has raised many questions, and clinical teams find themselves questioning whether it is necessary to initiate a clinical investigation, an observational study, or a survey to comply with the requirements for a PMCF study design.
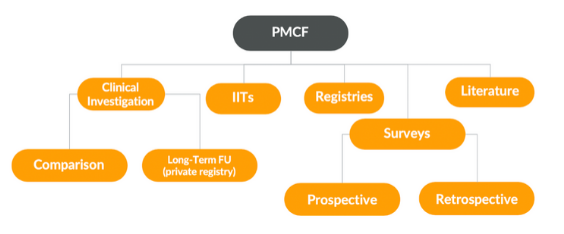
Image 1 - There is a variety of activities such as a PMCF Survey that can assist with PMCF data collection
A PMCF survey can be a less expensive alternative to a clinical investigation, but a PMCF survey also has its limitations. If your team has decided on conducting a PMCF survey, there are four things you need to keep in mind when creating the PMCF survey design, to ensure that your data will pass the scrutiny of notified bodies.
Check out our Practical Guide to PMCF EU MDR Compliance to learn more about other viable PMCF methods.
Below are our tips on how to design a PMCF survey.
1. Determine the Appropriate Sample Size
If your PMCF study design is to pass the scrutiny of Notified Bodies, it must contain justification (clinical and statistical) of the sample size. If you include too few or too many participants in your PMCF survey it will affect the accuracy and relevance of the results. As such, it will impact the usability of the data for the PMCF Evaluation report and regulatory compliance.
To calculate the appropriate sample size for your PMCF survey, you need to have knowledge of the expected results. This is used to determine how the study should be conducted, what data should be collected, and how its results should be analyzed (and statistically tested).
For most clinical teams, this should come naturally, because medical devices have been designed to serve a specific purpose, i.e. they have a clear intention of use and clinical claims. As such, a medical device manufacturer should have an idea of how the device will contribute to a clinical pathway, thus, have a hypothesis on how the device could affect the PMCF survey results.
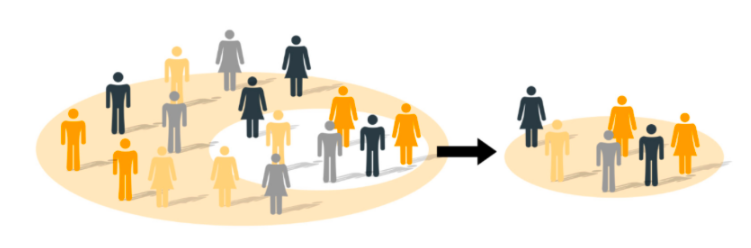
Image 2 - Determining the appropriate sample size for your medical device PMCF survey is a vital requirement for a PMCF plan
There are multiple techniques to determine the sample size for clinical studies and surveys, but they all have one thing in common: to find the smallest sample size necessary to detect a clinically relevant effect or difference.
Commonly, this is done by using mathematical equations derived from statistical tests, where you define and make assumptions on inputs that mirror your PMCF study goal, design, and clinical outcomes of interest, to calculate the appropriate sample size.
Download the Medical Device Sample Size cookbook and learn the process behind medical device sample size calculation.
2. Use Questionnaires that Provide Reliable and Usable PMCF Data
There’s a variety of validated questionnaires (or scales) available which can be used to gather data on different research topics. Validated questionnaires are especially useful when you need to gather data for regulatory purposes because they are scientifically tested.
This means that the results from those questionnaires can provide reliable information on the topic of interest - if applied correctly. In addition, it also simplifies the justification of your data collection method, to why you are asking specific questions.
From our experience, creating custom questionnaires and forms to use in a PMCF survey or clinical investigational context, often returns a more complex dataset.
This is another reason why standardized questionnaires are helpful. Because they provide sponsors with a structured dataset that is easy to analyze. It minimizes the need to clean data or merge open-ended or text-based answers. In the end it can save a great amount of time.

Image 3 - Well-structured data simplifies the Post-Market Clinical Follow-up survey analysis
With that said, we have to acknowledge that sponsors always need to define some custom questionnaires to use, as a PMCF survey will require device-specific data, which can be difficult to collect by solely using standardized questionnaires.
PMCF teams should follow the principles behind a well-designed eCRF and use the Greenlight Guru Clinical eCRF template as a structure for designing all questionnaires for the PMCF survey.
3. Use a Validated Tool to Simplify GCP Compliance
One of the critical quality requirements for both Clinical Investigations and PMCF under the MDR is compliance with GCP. To ensure that the data collected in a PMCF survey complies with the quality standards of the EU MDR, sponsors have to use solutions that can fulfill the ISO14155 requirements on electronic data capture.
This means that if you use survey software that does not comply with ISO14155, you risk not being able to use the PMCF survey data in your clinical evaluation report. Which then can potentially lead to a loss of CE mark.
Make sure to evaluate the survey software solution correctly, and ensure that you can:
-
Provide documentation of verification and validation according to the requirements specified in ISO14155:2020 section 7.8.3
-
Document data traceability from source to sponsor
-
Comply with all standard GCP principles when collecting, viewing, editing and exporting data
-
Store the data according to industry standards on general security and permission control
-
Comply with GDPR requirements for informed consents and data protection
4. Select a Tool that Provides Multiple Ways to Initiate PMCF Surveys
Traditional survey software is used to gather information from a specific user at a single time point. This is helpful if the data you are collecting is limited in size, complexity and you already know who you are going to survey.
But when it comes to PMCF surveys, gathering data on clinical performance and safety is a bit tricky. Especially because you do not always know who your survey participants are.
Additionally, clinical data often requires information to be collected on multiple clinical cases, and not necessarily the survey participant himself. This might, for example, require the same physician to participate in the same PMCF survey, multiple times. This is often referred to as “Cohort Studies” or “Case series”.
In the case of designing PMCF activities such as a PMCF survey for lower class devices and WETS, you would need to implement modern methods to gather data on clinical experience and safety. Listen to Evnia’s CEO, Efstathios Vassiliadis, in a joint webinar with Greenlight Guru Clinical where he offers his key insights on how to best collect PMCF data for lower class devices and WETs. Watch the webinar, for free, here.
Traditional survey software is not designed to accommodate that the respondents might have to be observed in time, which can place a limit on the data collection possibilities. MedTech clinical teams also tend to request more information than originally anticipated, which often results in larger datasets.
This means that if you’re going to request data on multiple cases via a survey, you need to evaluate the possibility of splitting it up into smaller phases or initiate the request for data collection in other ways. Else, you will risk not collecting any PMCF data at all, because you’re placing too much work on your participants.
Lastly, it’s not always possible to gather a list of emails of potential participants beforehand. Often you have distributors or other actors that cannot provide you with such information beforehand, due to e.g. GDPR. So, in order to collect any data, you need to be able to provide “ad-hoc” access to your survey as well.
Easily create PMCF surveys with Greenlight Guru Clinical
Need Assistance with PMCF Surveys? Greenlight Guru Clinical has many years of experience assisting MedTech companies with their PMCF surveys and PMCF study designs.
Reach out to our experts and book a free customized demo to see how Greenlight Guru Clinical can simplify your PMCF plan.
Jón Ingi Bergsteinsson, M.Sc. in Biomedical Engineering, is the co-founder of Greenlight Guru Clinical (formerly SMART-TRIAL). He was also the technical founder of Greenlight Guru Clinical where he paved the way for the platform’s quality standards, data security, and compliance.
Read More Posts
The Medical Device Practical Guide to PMCF Requirements under EU MDR
Selecting The Ideal PMCF Activity
The Practical Guide to Post-Market Clinical Follow-up EU MDR Compliance
Get your free eBook PDF
Selecting The Ideal PMCF Activity
%20Activities/(cover)%20Ultimate%20Guide%20to%20Selecting%20the%20Ideal%20PMCF%20Activity.png?width=250&name=(cover)%20Ultimate%20Guide%20to%20Selecting%20the%20Ideal%20PMCF%20Activity.png)


.png?width=2318&name=Selecting%20the%20Ideal%20PMCF%20Activity%20(cover).png)








