FOCUSING ON COMPLIANCE IS THE PROBLEM,
NOT THE
ANSWER.
A QUOTE FROM
FDA'S CASE FOR QUALITY
"Medical device manufacturers can reduce costs and increase their profits by focusing on quality measures during medical device design and production. This quality improvement approach pays dividends in customer satisfaction and provides significant competitive advantages.
In simple terms, our review of device manufacturers that consistently produce high-quality devices identified that an investment in quality has long-term payoffs. Quality of medical devices is in everyone's best interest."
COMPANIES THAT FOCUS ON
COMPLIANCE
- Just get by
- Get through a single audit
- Remain liable for future audits
- View compliance as a necessary evil
- Get comfortable kicking the can
- Make decisions without the data
- Open up unnecessary risk
- Plug holes with resources
- React to fire drills
- Fly blind
COMPANIES THAT FOCUS ON
QUALITY
- Are more productive and innovative
- Receive fewer complaints, investigations, and open fewer CAPAs
- Have lower quality-related product costs than their competitors
- Instill customer confidence and loyalty that their devices will perform as intended
- Free resources that foster innovation and accelerate new product introduction
- Mitigate risk leading to higher business valuation
TO ILLUSTRATE THIS POINT
Just because a restaurant doesn’t get any health violations doesn’t mean they are making great food.
And it doesn’t mean that they will be in business tomorrow.
THE BEST MEDICAL DEVICE COMPANIES DON’T JUST FOLLOW THE RULES, THEY LEAD WITH QUALITY.
NEWSFLASH...
THIS ISN'T A NEWSFLASH.
What’s true – and obvious – for restaurants is also true for the medical device industry. With only a focus on compliance, nothing has changed or gotten better for decades. This is a systemic, industry-wide problem and it needs to change.
“FDA has continued to observe that the percentage of inspections calling for official action by FDA has remained static, with the same issues recurring frequently year after year.”
-FDA'S CASE FOR QUALITY
WAIT...HOW DID WE GET HERE? WE DIDN'T HAVE A CHOICE.
Why is managing your quality system so hard and painful? Why does it seem to work against you and not for you? Why does it make you feel your only option is to focus on compliance, not quality?
The tools we’ve had are fundamentally broken.
There's been zero innovation in over 20 years.
These tools have not let us focus on quality.
PAPER-BASED SYSTEMS WON'T CUT IT. But they might cut your finger.
You can never be 100% positive all the appropriate approval signatures have been captured in a paper-based system.
Documents that are stored in different physical locations and filing cabinets are not always accessible.
Providing full transparency and traceability throughout the entire product lifecycle is impossible.
Paper-based design history files (DHF) and risk management files are static and not maintained throughout the entire product lifecycle.
There is no way to link any QMS subsystem to other subsystems (i.e. documents, design controls, risk management, CAPA, etc.) since they are completely isolated from each other.
Paper-based systems are inefficient, ineffective, create many failure points, and can be easily avoided and manipulated.
Employees may not be using the correct or the most up-to-date version which can jeopardize product quality and create lots of regulatory risks.
LEGACY AND GENERAL PURPOSE EQMS’ FALL SHORT.
The conventional approach to managing quality processes through a series of Word and Excel files stored on file servers is a logistical nightmare. When something changes, so many documents and data fields can be impacted and are often missed. Ensuring that each file is current and correct creates daunting amounts of busy work for engineers.
It is impossible to link any QMS object to any other QMS object (design controls to risk, CAPA to risk, documents to design controls, audit to nonconformance, etc.) in a legacy system; therefore, you cannot easily update and maintain information across the system nor ensure full transparency and traceability throughout the process.
Giving legacy systems a facelift does not change the fact the underlying architecture is outdated. Supporting old technology and architecture puts undue pressure on you and hinders your progress, especially if you are attempting to maintain an on-premise solution.
General-purpose systems do not provide medical device industry-specific templates, so you need to make significant modifications and updates to bring the system into compliance with any existing medical device regulations, and you need to stay current yourself on any new regulations that impact your system.
When you are forced to start from nothing and create and configure your workflows, you are facing extremely long implementation times and burdensome processes. A form-based system that is highly customizable is almost impossible to validate. A change to a workflow or to the structure of a form are events that require re-validation and any validation in this type of environment can take months.
Part 11 is merely a checkbox and not viewed as a functional architecture and process. It is typically an afterthought in general-purpose solutions.
Requiring a CAPA to be opened every time you need to conduct a root cause analysis, even for a complaint or nonconformance event, is incredibly inefficient and is not how a medical device CAPA system should work.
AND THERE’S A LOT AT STAKE IF YOU DON’T EMBRACE QUALITY
YOUR EXECUTIVE TEAM CONTINUES TO FLY BLIND.
What is really going on in the quality and regulatory departments?
Why do we invest in quality but continue to have quality issues?
Why does it seem like we’re always reacting?
YOUR QUALITY TEAM ISN’T PROPERLY EQUIPPED.
When the data in you quality system is siloed, it's impossible to make completely informed decisions.
You substantially increase your risk when you don't have visibility and traceability throughout your entire QMS.
YOU DON’T HAVE A QUALITY PRODUCT.
If you only focus on compliance, you’re exposed to risk of adverse events that may tarnish your brand. You may not be putting a product out on the market that is innovative and meets market demands.
YOUR BOTTOM LINE IS IMPACTED.
THE MEDICAL DEVICE INDUSTRY MUST SHIFT FOCUS FROM COMPLIANCE TO QUALITY.
BUT HOW?
WE ARE GREENLIGHT GURU.
OUR MISSION IS TO IMPROVE THE QUALITY OF LIFE.
We are focused on making quality the core focus of your daily life – not pushing paper, tracking down signatures, trying to find files, or being surprised. You are doing what you do because you have a passion for helping people, and you have a desire to make a difference in the world.
“I can't even imagine passing the audit - or even surviving - if we had gone with a paper-based QMS.” - Gabriel Sanchez, CEO, Zebra Medical Technologies
You will no longer spend time doing non-value add activities but rather, spend time on helping your company make high-quality, impactful devices. We don’t just help the company, we help the person using our software get back to their why.
WE ARE THE INDUSTRY.
Our expertise is a result of our life's work. We offer in-house gurus with
10+ years of industry expertise as well as a worldwide partner network.
#1 BlogIn the industry |
75 YearsIndustry experience |
250,000Podcast listeners |
#1 WebinarIn the industry |
#1 PodcastIn the industry |
100,000+Rely on us for the |
DESIGNED BY MEDICAL DEVICE PROFESSIONALS FOR MEDICAL DEVICE PROFESSIONALS. BASED ON THE LATEST FDA AND ISO STANDARDS AND THE BEST PRACTICES OF MEDICAL DEVICE MANUFACTURERS THAT CONSISTENTLY PRODUCE HIGH-QUALITY DEVICES.
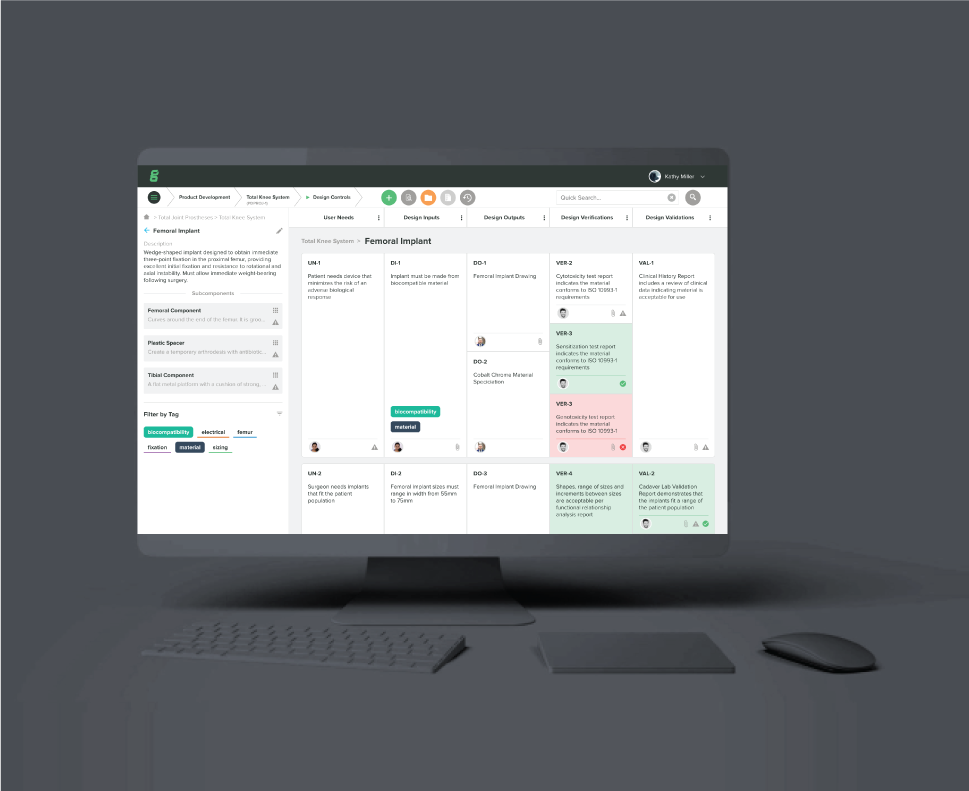
Achieve End-to-End Traceability
Easily trace CAPAs all the way back to your design controls so your employees can focus on accelerating your business.
Automate Quality Processes
Integrate quality and risk management into your design and development processes from the start.
Follow FDA/ISO Best Practices
The only software with the latest FDA and ISO best practices specific to medical device companies built into every feature.
Streamline Team Communication
Communicate where your team works. Avoid unecessary meetings, emails and communication across apps.
- 21 CFR Part 11 Compliant
- Workflows aligned to FDA and ISO standards for medical device predefined processes
- FDA 21 CFR Part 820
- ISO 13485:2016
- ISO 14971:2019
SOFTWARE FEATURES
DESIGN CONTROL - Automatically maintain full traceability
DOCUMENT MANAGEMENT - Easily access up-to-date documents
RISK MANAGEMENT - Gain new visibility and mitigate risk
CAPA MANAGEMENT - Identify and address issues faster
AUDIT MANAGEMENT - Conduct audits with ease
COMPLAINT MANAGEMENT - Streamline feedback and complaint processes
NONCONFORMANCE MANAGEMENT - Reduce cycle times and associated risks
CHANGE MANAGEMENT - Understand and manage the impact of any change
TRAINING MANAGEMENT - The right training to the right people at the right time
-2.png?width=500&height=501&name=GG-LinkedIn-profile-pic-green-1%20(1)-2.png)

