QMS for SaMD
Bring Your Software Innovation
to Life
Software as a Medical Device (SaMD) is one of the fastest-growing segments of the medical device industry, creating a new future of patient experience and care. But with rapid advancements and constant innovation, the SaMD landscape is changing quickly. Let us be your guide in delivering new innovations to patients.
 Calculate Your ROI
Calculate Your ROI
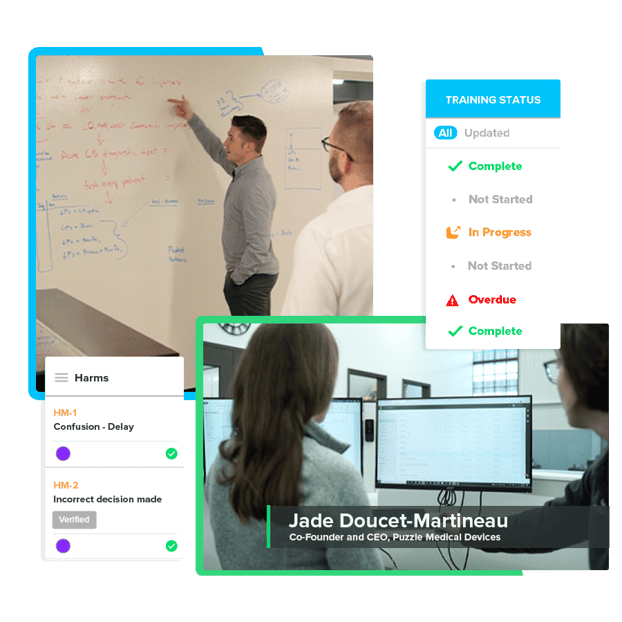
Software Supporting Software
In addition to keeping up with a constantly evolving regulatory landscape, SaMD manufacturers must also effectively and efficiently optimize their software development practices to meet QMS requirements. But due to the speed of changes, there are often gaps in knowledge about global regulatory practices. Today's digital age demands a purpose-built solution for SaMD companies that performs better than disconnected general-purpose tools.
You’re in Good Company
Join some of the most exciting and innovative thinkers in the MedTech industry.









Built for Emerging Medical Technologies Like Yours
Pull together the power of your teams with our end-to-end solution to manage product development, quality, and suppliers. Watch your devices come to life and have confidence in the work you’ve invested in them.
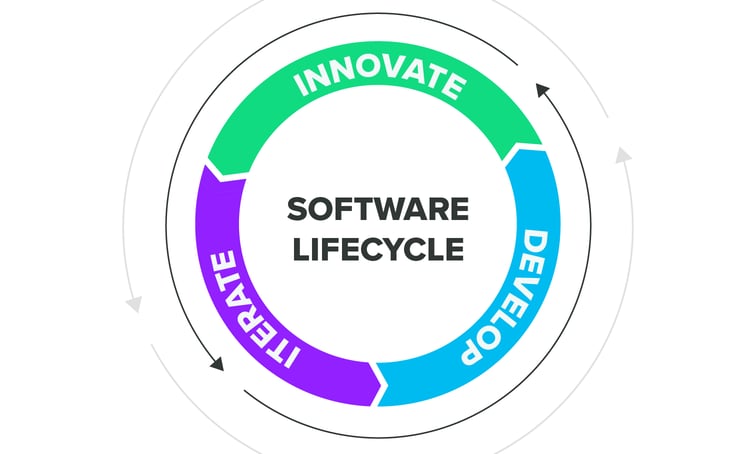
Innovate. Iterate. Develop. Repeat.
Support an agile product development approach and enable your team to work dynamically and efficiently throughout the process by leveraging a platform that promotes transparency and cooperation rather than operating within disconnected silos. Instill risk-based processes to be carried out throughout the entire medical device software lifecycle and ensure compliance with IEC 62304.

Connect Design to Development
Enable software development teams to leverage the agile functionality of JIRA when building software applications or components.
- Give your team the freedom to execute work in JIRA.
- Navigate seamlessly between linked design controls and associated JIRA issues.
- Achieve visibility between your design control and risk management system of record and the software implementation being tracked in JIRA.

Navigate the Open Waters of Regulations
With governing authorities still developing their own regulatory controls, manufacturers have new opportunities to shape the present and future landscape. At the same time, SaMD companies can control the unknowns and reduce barriers to compliance with our purpose-built QMS software that functions beyond general-purpose tools.
Safe and Secure Software
Identify problems before they become disasters. Today, officials monitor cybersecurity measures for SaMD. A software bill of materials is one of the most powerful tools in your arsenal.

Innovate. Iterate. Develop. Repeat.
Support an agile product development approach and enable your team to work dynamically and efficiently throughout the process by leveraging a platform that promotes transparency and cooperation rather than operating within disconnected silos. Instill risk-based processes to be carried out throughout the entire medical device software lifecycle and ensure compliance with IEC 62304.

Connect Design to Development
Enable software development teams to leverage the agile functionality of JIRA when building software applications or components.
- Give your team the freedom to execute work in JIRA.
- Navigate seamlessly between linked design controls and associated JIRA issues.
- Achieve visibility between your design control and risk management system of record and the software implementation being tracked in JIRA.

Navigate the Open Waters of Regulations
With governing authorities still developing their own regulatory controls, manufacturers have new opportunities to shape the present and future landscape. At the same time, SaMD companies can control the unknowns and reduce barriers to compliance with our purpose-built QMS software that functions beyond general-purpose tools.

Safe and Secure Software
Identify problems before they become disasters. Today, officials monitor cybersecurity measures for SaMD. A software bill of materials is one of the most powerful tools in your arsenal.










