top takeaways
.png?width=65&name=1%20(2).png)
20% of companies have had product development halted due to incomplete risk management
.png?width=65&name=2%20(1).png)
27% of product submissions were delayed due to inadequate risk management

The average delay due to risk management mistakes was 5 months
Understanding the risk management landscape
In the Greenlight Guru 2021 Risk Management Benchmark Study, just over half of those surveyed (53%) say risk is considered a strategic asset inside their organizations. Perhaps not surprisingly, senior executives are more likely to say their organizations treat risk as a strategic asset than do those who work in quality or product development exclusively.
For people who work in risk management day-to-day, there’s frustration that some don’t treat the discipline with the care it requires. As one respondent shared, “The design and development team are not taking on board that risk is intrinsically linked to the process, and it has to be accounted for and documented to ensure we can fully evaluate the device we will be selling in the future.” Another survey taker shared, “Others in the organization do not engage in activities that open up communication about daily activities and ongoing discussions of risks, so as a compliance officer I can't be proactive.”
Get your free copy of
2021 Risk Management Research Report

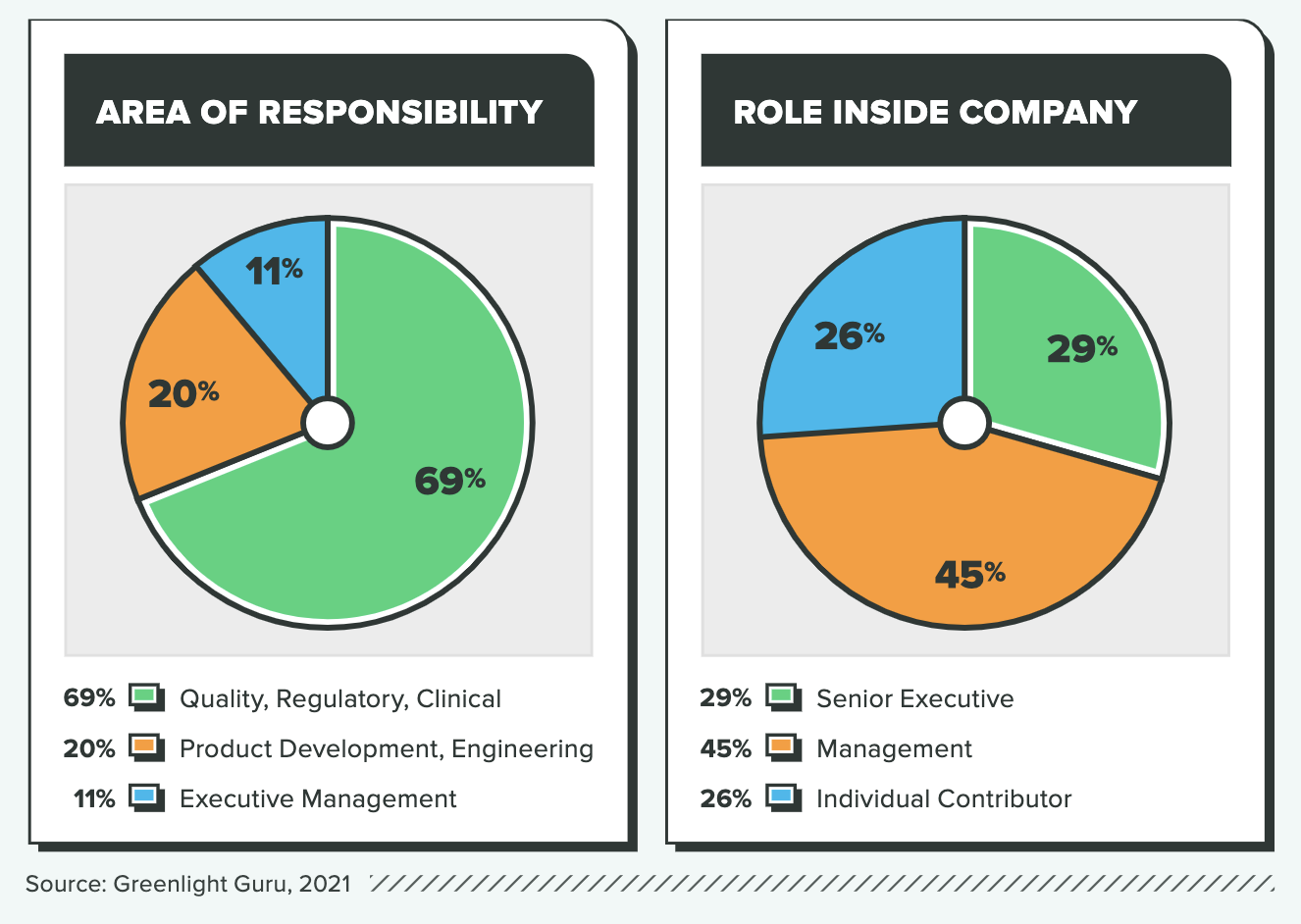
WHO TOOK THE SURVEY
The results in this report are from an online survey that was fielded from April 19 to May 9, 2021. A total of 363 global respondents completed the survey. Key demographic variables are included below.
Risk Management Mistakes Lead To Costly Delays
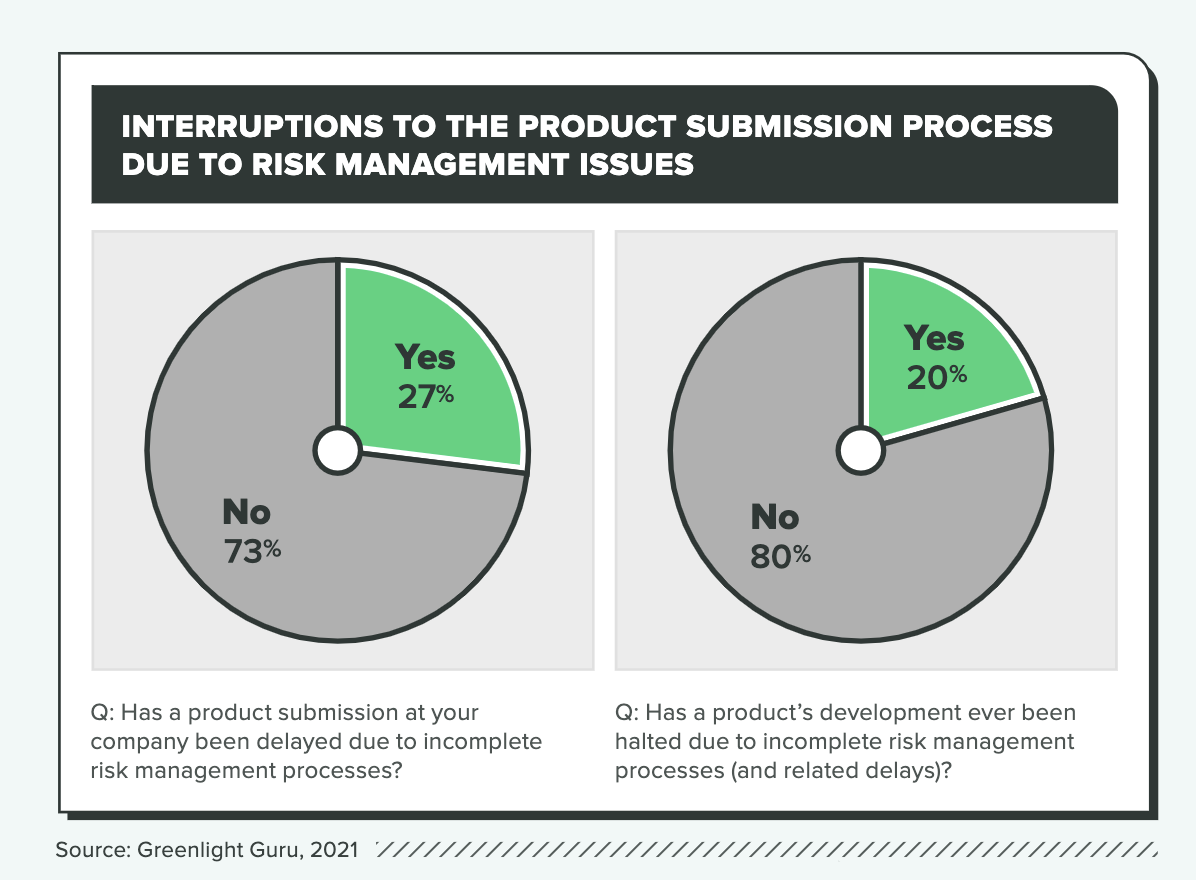
Ineffective risk management practices can delay or even derail product development, and according to the research, 1 in 3 companies that treat risk management as a “checkbox” activity report delays due to faulty risk management. (The ratio is a little more than 1 in 4 across all companies.)

The real costs associated with risk management missteps
Those disruptions can cause delays of up to a year or more, but the average is 5 months. If we assume a burn rate of $400,000 per month, which would be reasonable for a medical device startup with VC funding, the cost of incomplete risk management practices would be $2 million on average, and some would spend well in excess of that number on delays.
Risk Management Impacts Morale
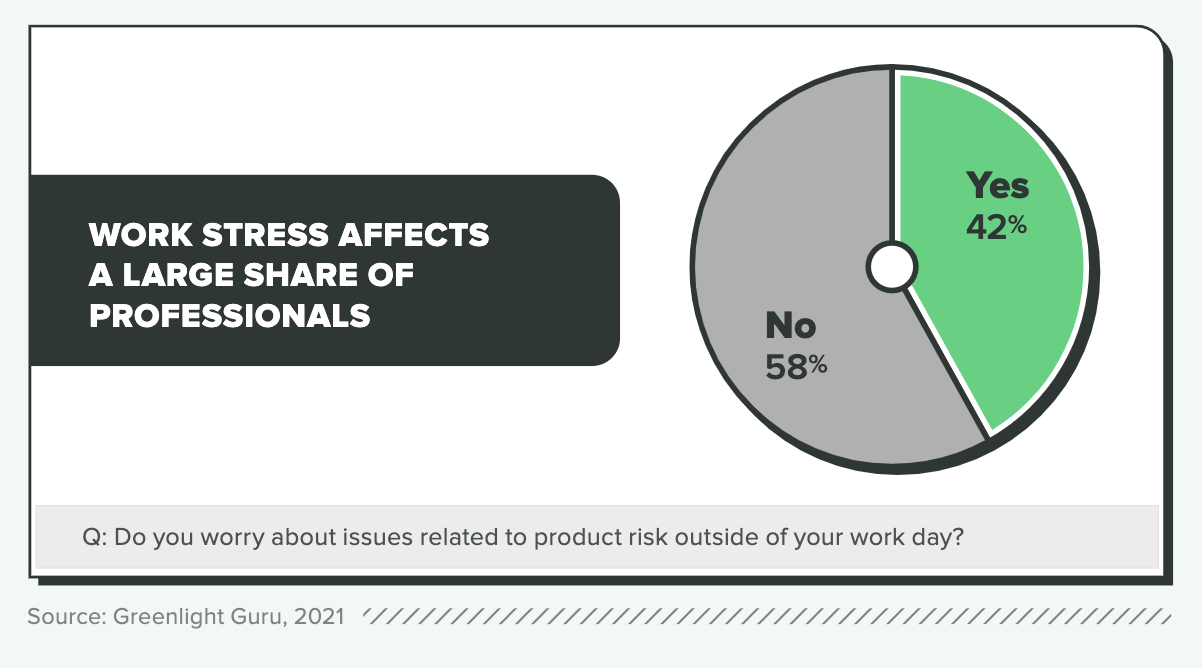
In addition to the financial penalties, there are also human risks related to employee stress levels and well-being. Of those surveyed, 42% say they worry about issues related to product risk outside of their work days, and well over 1 in 4 (29%) say it has a moderate or serious impact on their quality of life.
Data Silos Lead to Missteps
Siloed data, says Sebastian Liedtke of Roche Diabetes Care, can be a challenging artifact of outdated organizational structures, particularly among medtech companies. Liedtke explained to McKinsey, “Historically, many medtechs have been technology-centric rather than user-centric. As a result, they are structured in tech silos: mechanical, electrical, software, and so on. Each of these silos has optimized its own individual part rather than addressing the entire patient journey.”
|
|
40% say data silos are a moderate or severe problem for their organization |
|
|
Senior executives were over 2x more likely than individual contributors to see data silos as an issue in their organization |
|
|
19% of respondents said the feedback loop between post-production and risk management was ineffective |
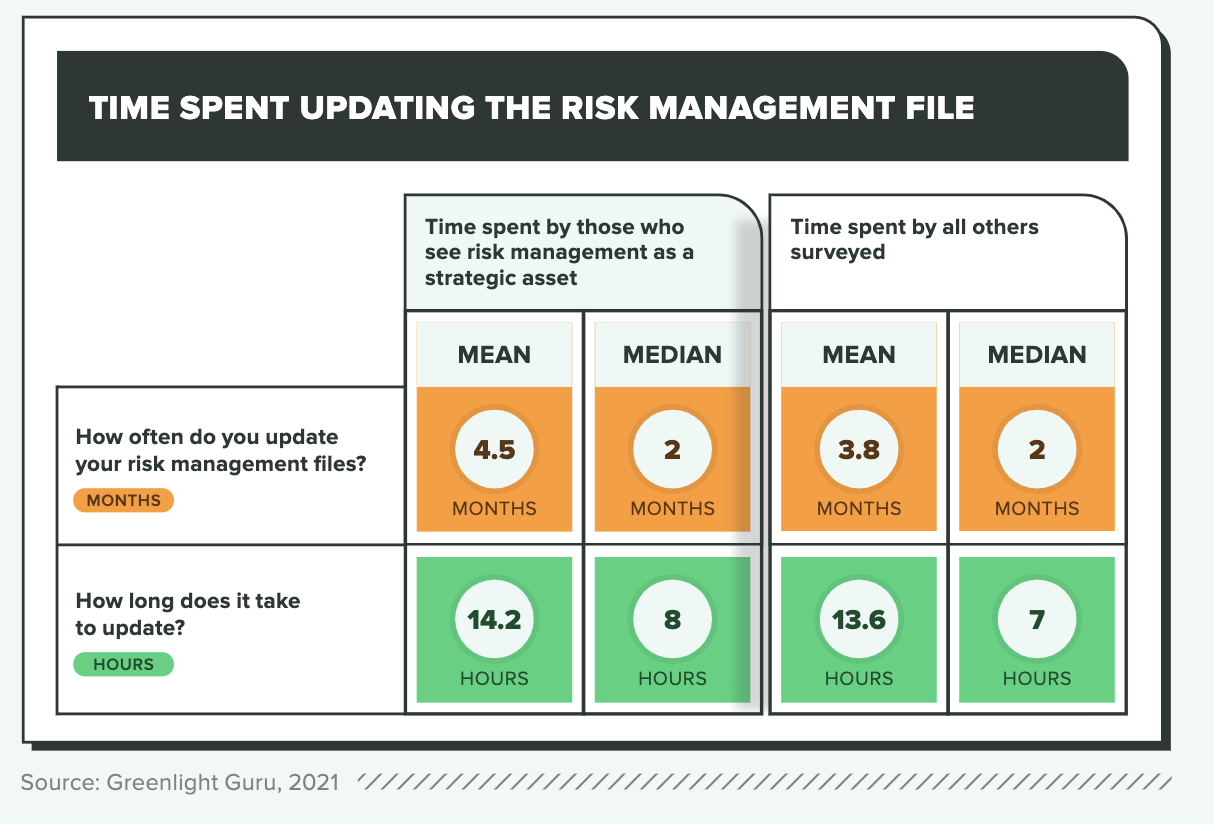
What sets the most effective risk management apart from the rest?
Risk management is a challenging discipline because when it’s done well, it’s largely invisible (and according to some survey respondents, unappreciated). As one survey-taker explained, the hardest part of creating dynamic, effective feedback loops is “getting organizational attention.” Another admitted, “My company has not embraced a quality culture. Silos exist and interfere in the closed-loop feedback structure from product development, production, and post-market surveillance. There are only two people in the company that understand this (me being one of them). The worry is that something will fall through the cracks and blame will be assigned to an individual, versus the culture or the system.”
How can you manage risk more effectively?
DOWNLOAD THE FULL REPORT TO UNCOVER what sets the most mature players apart from the rest.



.png?width=232&name=Report%20Download%20CTA%20(1).png)


