Risk Intelligence For MedTech
AI-powered Insights to Revolutionize Risk
Greenlight Guru’s Risk Intelligence provides AI-generated insights from industry databases so MedTech teams can proactively identify, document, and mitigate risk with more accuracy throughout the device lifecycle.
See the Demo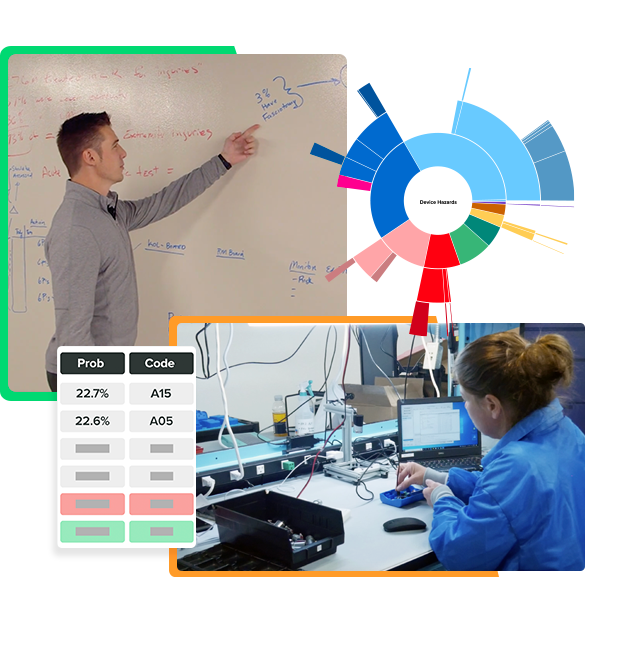
A New Approach to Risk Management
Assessing risk can be a challenge due to inconsistent data and limited access to research. This can lead to uncertainty and gaps in your assessment of device hazards and patient harms and probabilities of occurrence.
Greenlight Guru’s Risk Intelligence provides the necessary data to improve your work and make informed decisions quickly.
First-of-its-kind Intelligence for Risk
Greenlight Guru’s Risk Intelligence leverages AI and proven statistical models that help you predict the most relevant risks for your devices.
This will save lots of time normally spent brainstorming, researching, documenting hazards and patient harms, and trying to estimate probabilities and severities. I love that Risk Intelligence lets us combine our existing expertise with AI-driven insights derived from FDA databases to best evaluate risk for our device.
Brad Cunningham
Director of Quality Assurance | Pirouette Medical
Drive Effective, Efficient, and Confident Risk Management
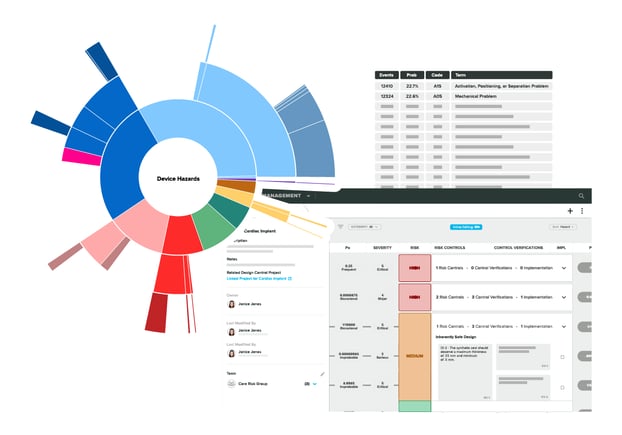
Risk Solutions for MedTech
Building and evaluating Risk across the device lifecycle is a challenging process. Accelerate this crucial task by integrating Risk Intelligence with your Risk Management.
Make informed, data-driven decisions to optimize risk management throughout your device lifecycle.
Learn More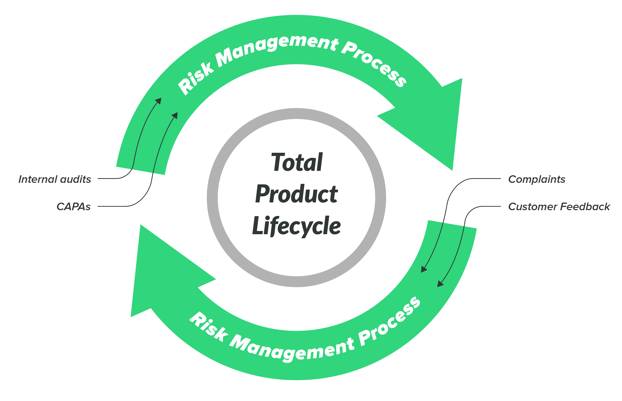
Integrated and Compliant Risk Management
Greenlight Guru provides you with an ISO-14971-aligned Risk Management workspace that enables complete traceability with the rest of your QMS.
Integrate Risk-based thinking into your entire device ecosystem, keeping you in compliance with ISO 14971:2019 and the risk-based requirements of ISO 13485:2016.
Learn More
















.png?width=425&height=213&name=Spring%2023%20Badges%20(1).png)