
Many medical devices include some type of software in the device. Industry standards define that the software components be managed separately just like the hardware and software components of traditional medical devices.
Manage each individual module or component of a software component or device through its own design workflow and ensure that each component is reviewed and approved as part of the whole.
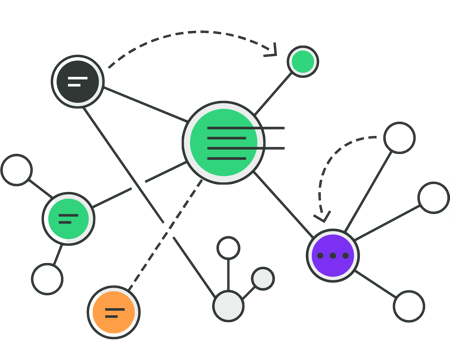
See your device's design in a single, enhanced view.
Visualize the connections throughout your device requirements and QMS to better understand the shared relationships between items. See shared specifications across components and have insight into manufacturing or supplier issues.
Easily navigate between test reports and protocols to your design control verifications and validations, while also seeing related design inputs, risk controls, and components.
.png?width=2400&name=mdQMS%20chart%20(5).png)
![G2 crowd [general] badge](https://www.greenlight.guru/hs-fs/hubfs/G2%20crowd%20%5Bgeneral%5D%20badge.png?width=325&name=G2%20crowd%20%5Bgeneral%5D%20badge.png)
According to G2's Winter 2021 Report, Greenlight Guru is yet again a quality management software Leader, based on high customer satisfaction scores and its large market presence.
For nine consecutive quarters, Greenlight Guru has been recognized as the leader in Quality Management Software Solutions. Download the free report from G2:



