Schedule your free demo
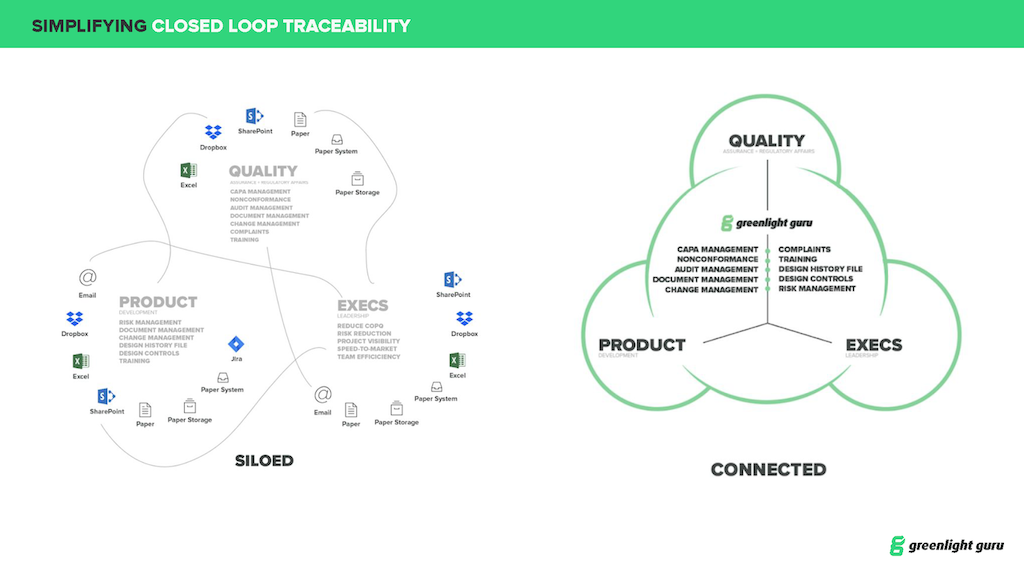
A Connected Quality Ecosystem
That Unites Your Whole Team
.png?width=2400&name=mdQMS%20chart%20(5).png)
THE ONLY QUALITY MANAGEMENT SOFTWARE DESIGNED SPECIFICALLY FOR THE MEDICAL DEVICE INDUSTRY


.png)


.png)
.png)

EASY TO USE, FAST TO IMPLEMENT, MODERN QUALITY MANAGEMENT SYSTEM THAT CONNECTS DISPARATE PROCESSES, SOURCES, PEOPLE, AND DATA FOR THE FIRST TIME EVER. INCREASED VISIBILITY SMOOTHS YOUR PATH TO COMPLIANCE AND LETS YOU FOCUS ON TRUE QUALITY.
- Achieve End-to-End Traceability
- Automate Quality, Risk, & Design Processes
- Follow FDA & ISO Best Practices
- Streamline Team Communication
Discover the power of end-to-end traceability ↓↓
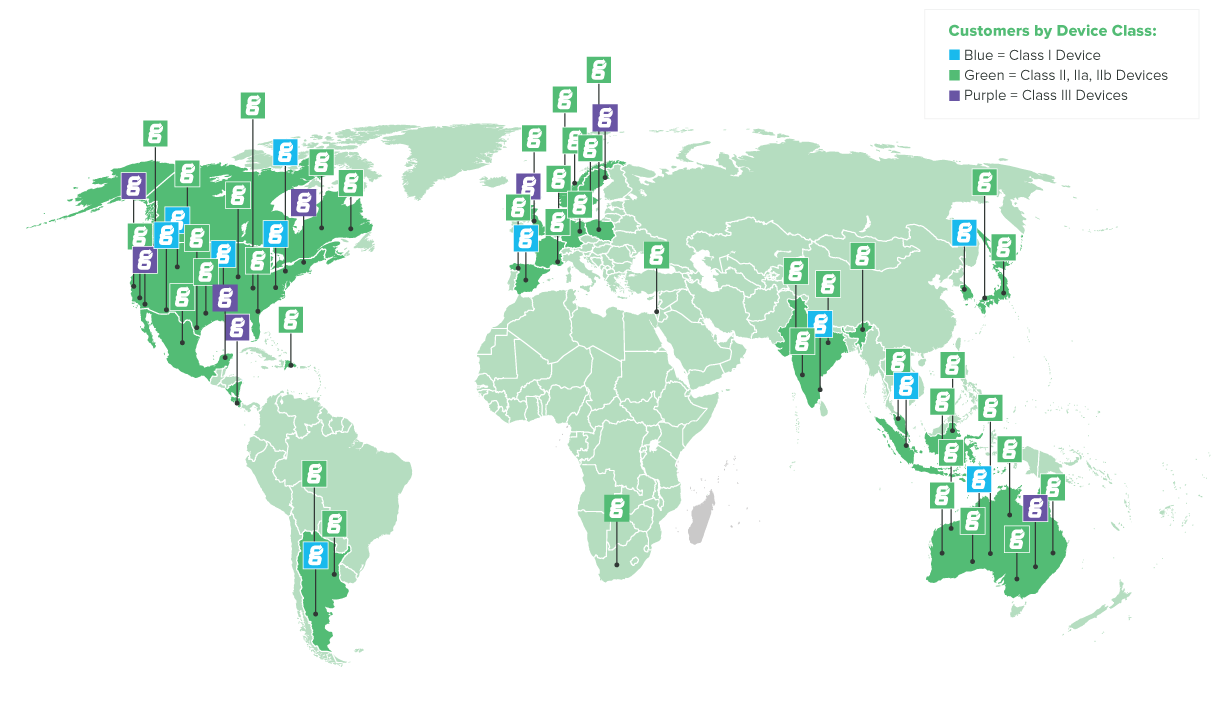
About Greenlight Guru
| Started in 2013 by medical device industry veterans | |
| Only True Cloud QMS with fully connected Design Controls & Risk | |
| Headquartered in Indianapolis, IN | |
| Hundreds of customers on six continents |
SOME OF THE MOST INNOVATIVE MEDICAL DEVICE COMPANIES IN THE WORLD IMPLEMENT THEIR QUALITY CULTURE USING OUR SOFTWARE


I was actually a little nervous going into the audit, because it seemed too effortless. I've worked in QA at a Fortune 500 company with a custom solution. Your flow is better. It saves time and it doesn't break up my thought process. With Greenlight Guru I'm able to focus on quality.


I believe the quality system I've instituted actually gives me a competitive advantage against a lot of the large companies I used to work for.


As a design firm, our ISO 13485 certification is a competitive advantage. Greenlight Guru made getting it easy. Our designers aren't accustomed to being under a quality system, but your simple interface made it possible to adopt. It's actually working.




Your software helped us tremendously in the last few months of 2017, during which we had an important ISO 13485:2016 audit. This obviously reflected on the auditors: it was very easy to show them in what phase every project was, but also to simply show every piece of documentation we have. In the auditors conclusion...




I've been thrilled with my experience working with Greenlight Guru. I am new to quality systems and compliance, and Greenlight Guru has made this transition very easy. The well-organized user interface and tracking features make the software simple and worry-free; I genuinely recommend it to anyone in the indus...


Using the Greenlight Guru system has enabled us to better implement our quality system across the company and accelerate our product development.


Greenlight Guru has made the design control and risk management process extremely easy to understand and explain to people not familiar with the process and helped them to understand how all of the steps are linked. It has also made it significantly easier to get documentation reviewed and approved.


We adopted Greenlight Guru 18 months ago to build our QMS. We recently passed our ISO 13485 Stage 2 audit, due in part to the ability to demonstrate a comprehensive matrix of risk and design controls. Demonstrating our QMS using a tool like GG was fundamental in this achievement. I'll also say that the team at ...


We've been using Greenlight Guru for nearly 5 years now and it really simplifies quality management. It's very easy to get all of our team on the same page and effortlessly trace various efforts through the system. The Greenlight Guru team is one of the best I've ever worked with. They bend over backw...


When it comes to the way they have designed the fully integrated workflows exclusively for medical device and continue to innovate with new releases, I'd call them the Tesla of medical device eQMS software.


Having 20 years of medical device experience, I have worked with large and elaborate design controls and small and lean design controls. I have also seen the disaster resulting from the absence of appropriate design controls. Greenlight Guru is exactly big enough to ensure compliance but small enough to be easily ...


This is my first time going through a medical device product development process. I've heard about all the challenges with Design Control. With Greenlight Guru, I know what to do and when to do it. Documenting Design Controls is really easy with this software.




A lot of times, if people don't understand quality systems, they think they just want to use paper because it presents as the cheaper option, but they fail to evaluate the total cost of ownership and impact on the business. The biggest differentiator was that it was all web based and didn't need any custom...








DERIVE VALUE IN YOUR FIRST 30 DAYS WITH GREENLIGHT GURU
With Greenlight Guru, you can launch new devices faster, streamline quality, and simplify compliance with a single source of truth that unites the team.
Greenlight Guru simplifies quality management and compliance. We keep our solution packages simple, too.
Our software packages are built to make it easy for you to adopt our platform. The difference amongst the packages is based on the needs of your team and business at different stages of your companies growth.
WHAT YOUR TEAM CAN EXPECT WITH GREENLIGHT GURU
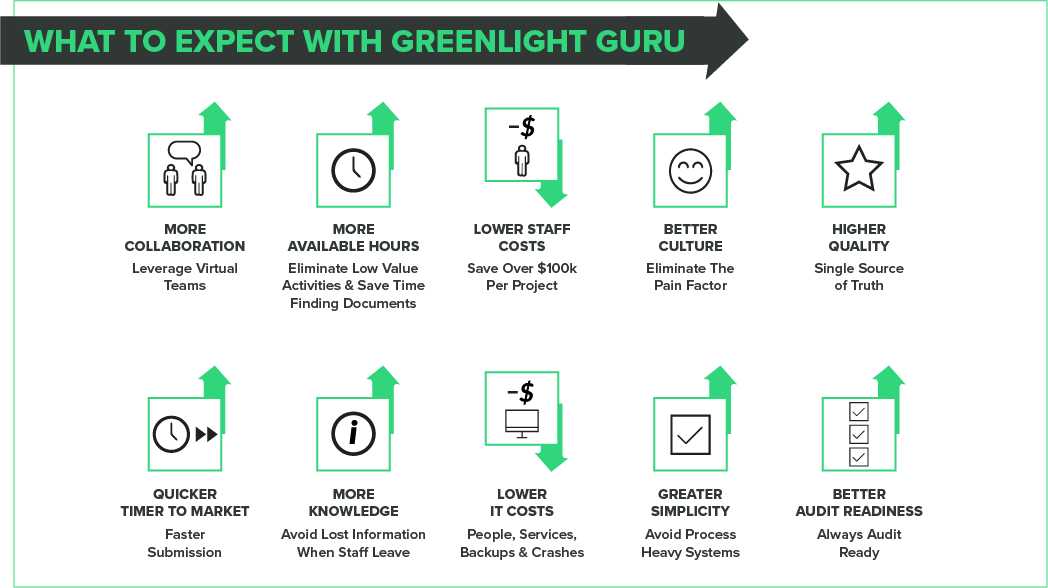




- $125,000 saved on average cost estimated per project
- Improved culture during project cycle
- 2.5 months faster to market
- 402 hours saved on low value-added activities
- Single source of truth for all quality processes
- Better audit readiness
INNOVATIVE MEDICAL DEVICE COMPANIES LIKE YOURS FIND SUCCESS WITH GREENLIGHT GURU
 Greenlight Guru has been instrumental for us while we efficiently navigate the quality management system process and with developing our FDA Submission.
Greenlight Guru has been instrumental for us while we efficiently navigate the quality management system process and with developing our FDA Submission.
Ryan Nolan
Co-Founder & VP of Clinical Operations PhotoniCare
 We wanted a QMS that was robust enough to grow with us, but simple enough to get us started.
We wanted a QMS that was robust enough to grow with us, but simple enough to get us started.
kevin mcleod
ceo at c2dx, inc
RESOURCES TO HELP YOUR DEVICE COMPANY MOVE BEYOND BASELINE COMPLIANCE TO TRUE QUALITY
BEST QMS SOFTWARE: ULTIMATE GUIDE TO COMPARING QUALITY MANAGEMENT SYSTEM SOLUTIONS |
|
15 QUESTIONS TO ASK QMS SOFTWARE VENDORS IN THE MEDICAL DEVICE INDUSTRY |
|
5 DO'S AND DON'TS WHEN CHOOSING A QMS SOLUTION FOR YOUR MEDICAL DEVICE COMPANY |
| The best QMS software comparison guide for medical device companies that will help you choose a quality management system solution that strategically benefits your product and company. | Know what questions to ask software vendors that give your medical device company the necessary information to consider when selecting a QMS software solution. | A guide to the top 5 recommended best practices that medical device companies should consider when choosing a quality management system (QMS) solution. |
 |
 |
 |
A COMPLETE GUIDE TO BRINGING A MEDICAL DEVICE TO MARKET |
|
ISO 13485 - ULTIMATE GUIDE TO THE QUALITY MANAGEMENT SYSTEM (QMS) FOR MEDICAL DEVICES |
|
DEFINITIVE GUIDE TO ISO 14971:2019 RISK MANAGEMENT FOR MEDICAL DEVICES |
| A complete guide for medical device manufacturers looking for guidance on how to plan for a successful product launch and maintain regulatory compliance across global markets. | Greenlight Guru eQMS platform automates regulatory compliance with ISO 13485:2016, FDA, ISO 14971, so companies can focus on true quality of devices. | Learn the most up to date recommendations and best practices from the ISO 14971:2019 standard and how you can start to use risk management as a tool - not a checkbox activity. |
 |
 |
 |





![Customer Onboarding and Training Guide [2020]_Page_13](https://www.greenlight.guru/hs-fs/hubfs/Customer%20Onboarding%20and%20Training%20Guide%20%5B2020%5D_Page_13.png?width=3000&name=Customer%20Onboarding%20and%20Training%20Guide%20%5B2020%5D_Page_13.png)





