Medical Device Project Management Software
Enhance Project Management For Your Teams
Stop using complex and disjointed tools. Spend more of your time focusing on the innovation of your medical technologies for patients who need them. Uncover better project management with our Design Control Software that keeps innovation, quality, and traceability at the forefront.

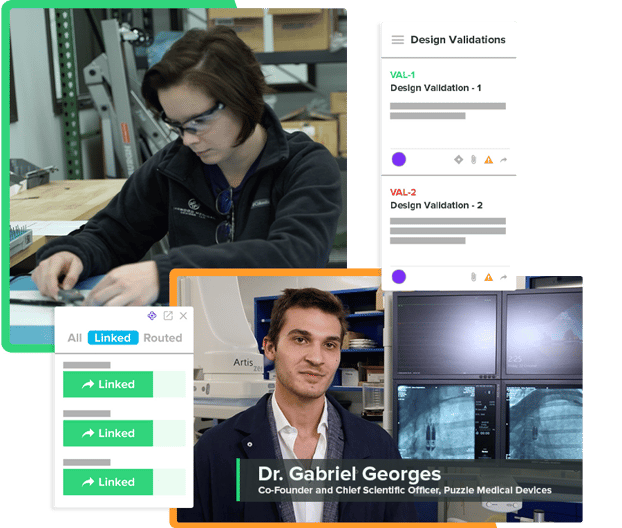
Data Silos Will Slow You Down
Achieving traceability using paper or other disconnected solutions can quickly become expensive and time-consuming. Your QMS should go above and beyond. With Greenlight Guru, you can drive collaboration on all ongoing project design and development activities, link any documents, conduct reviews of your projects, and generate a design history file—with a single click. Creating visibility and traceability between your quality events and project management processes will help you bring safer devices to market faster.
Project Management For Your Medical Devices
Leverage Greenlight Guru’s Design Control Software to manage your various device projects and document them all while staying compliant.
Powerful Project Management Doesn’t Need To Be Complex
Designed specifically for the MedTech industry with simplicity and flexibility in mind.
Collaborate and track design reviews with Part 11 compliant workflows.
Collaborate and track design reviews with Part 11 compliant workflows.
Create detailed design control objects, link complex configurations, and attach documents with one click.
Create detailed design control objects, link complex configurations, and attach documents with one click.
Generate design history files and records without searching for required documentation.
Generate design history files and records without searching for required documentation.
Prioritize ISO 14971 and ensure compliance while you design your devices.
Prioritize ISO 14971 and ensure compliance while you design your devices.
Benefits for Teams
For Development Teams
Streamline design processes so you never miss a step
- Spend more time designing and less time doing paperwork
- Collaborate with your R&D team on verification and validation testing
- Link all relevant documents, specifications, and design components together with a single click
Benefits for Teams
For Quality Teams
Maintain true quality, traceability, and visibility with every project
- Bring a safe, compliant device to market with a solution built specifically for the MedTech industry
- Easily achieve 21 CFR Part 820.30 and ISO 13485 compliance with an auto-generating, auto-updating DHF
- Prioritize ISO 14971 and access risk connected to design controls at anytime

Integrate with Jira
Enable product development teams to leverage the agile functionality of Jira while maintaining traceability to design control processes throughout the MedTech lifecycle.
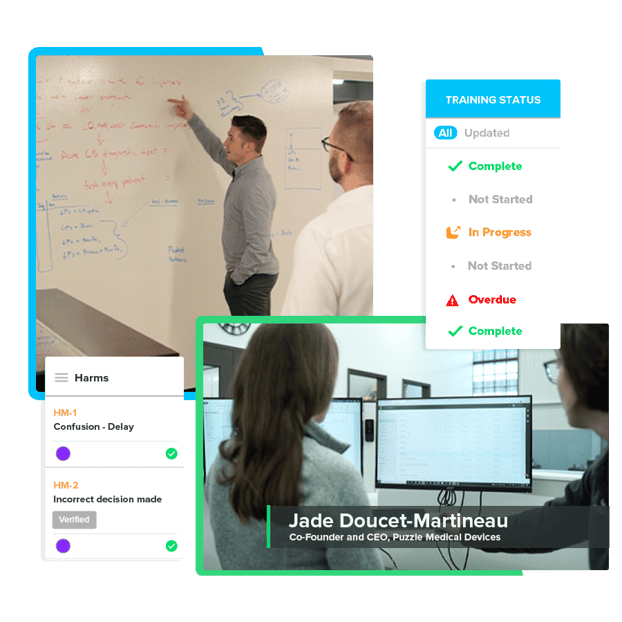
Software as a Medical Device
Industry standards require that the stand-alone software components be managed separately, just like the hardware and software components of traditional medical devices. Manage your software components and devices through their own design workflow, ensuring each component is reviewed and approved as part of the whole product.
















