Study building, data management, and security & quality
How to Get Started with Greenlight Guru Clinical
Everything you need to know about onboarding, study building, data management, security, and quality to ensure compliance with ISO 14155:2020, Good Clinical Practice (GCP), and other regulatory requirements.
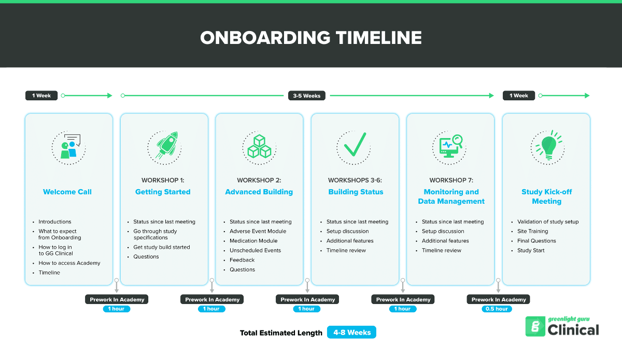
Greenlight Guru Clinical Onboarding Program
Our Onboarding includes consultation and recommendations on your clinical data management processes, and you will naturally learn Greenlight Guru Clinical basics and best practices for data management. We also provide templates to use in your internal clinical QMS. Onboarding includes:
- Form design
- Training in Study setup
- Introduction to Greenlight Guru Clinical regulatory templates
- Guidelines on external site training
- Guidance on compliance with FDA, GCP & GDPR
- Dedicated Account Executive & Customer Success Executive
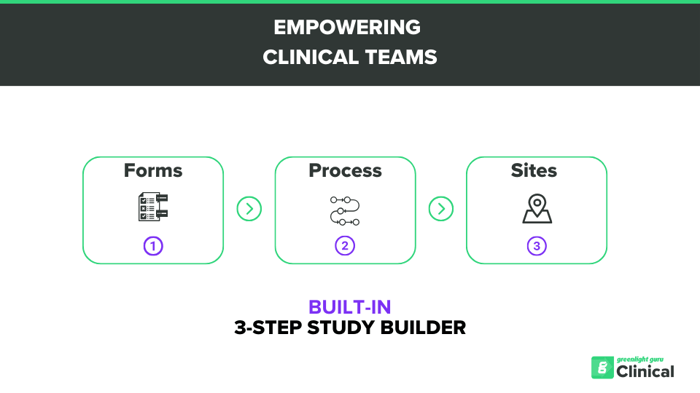
The Ideal Study Builder for MedTech
Map and visualize your unique data collection plan in Greenlight Guru Clinical
Your project starts with a question in a form:
- Intuitive drag-and-drop form creator
- eCRFs, questionnaires, and surveys
- Ready-to-use form validation
Your project starts with a question in a form:
- Intuitive drag-and-drop form creator
- eCRFs, questionnaires, and surveys
- Ready-to-use form validation
The process defines what and when forms shall be completed:
- Visit events for eCRF data entry
- Send forms/questionnaires via e-mail or SMS
- Fully automated or manual

The process defines what and when forms shall be completed:
- Visit events for eCRF data entry
- Send forms/questionnaires via e-mail or SMS
- Fully automated or manual
After creating a process for your clinical data collection, create your site or sites and link them to the relevant Process for the individual site:
- Unlimited no. of sites
- Role-based access control to each site
- Track progress and compliance for each site

After creating a process for your clinical data collection, create your site or sites and link them to the relevant Process for the individual site:
- Unlimited no. of sites
- Role-based access control to each site
- Track progress and compliance for each site
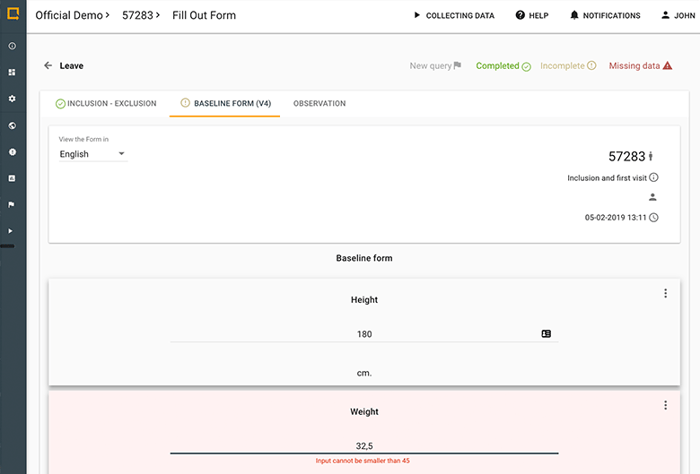
See how you can set up and build a Study in Greenlight Guru Clinical in only 90 seconds.
See how you can set up and build a Study in Greenlight Guru Clinical in only 90 seconds.




MedTech Compliant Data Management
Features to ensure GCP and ISO 14155:2020 compliance
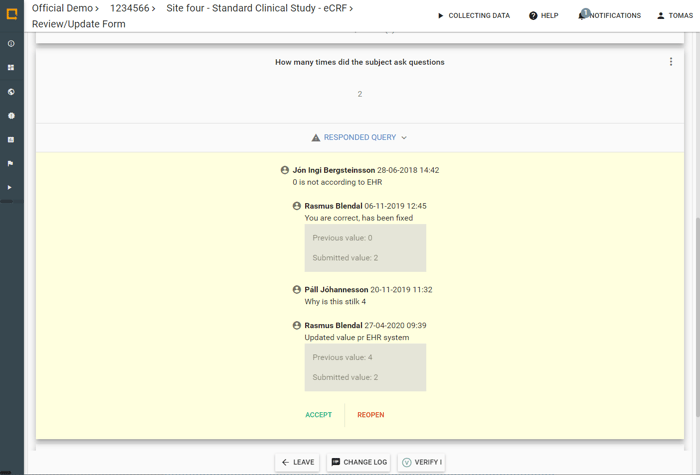
Converse on study endpoints with collaborators and communicate via Query Messages.
Full trail of corrections made to the data and a full overview of all Queries.
- Raise queries with validation rules
- Stay up to date with in-app and email notifications
- Detailed status of all queries in Study Query List
- Manual and automatic query functionality
Converse on study endpoints with collaborators and communicate via Query Messages.
Full trail of corrections made to the data and a full overview of all Queries.
- Raise queries with validation rules
- Stay up to date with in-app and email notifications
- Detailed status of all queries in Study Query List
- Manual and automatic query functionality
Improve data consistency and validity with Greenlight Guru Clinical's monitoring and verification types. “Verification I & Verification II”. Perform Source Data Verification, remote monitoring and site monitoring.
- Customizable Naming .e.g. Remote monitor
- Verify Forms or individual data points
- Permission based verifications
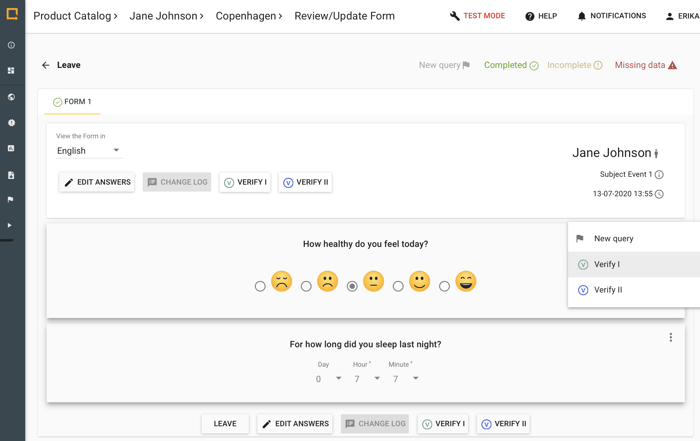
Improve data consistency and validity with Greenlight Guru Clinical's monitoring and verification types. “Verification I & Verification II”. Perform Source Data Verification, remote monitoring and site monitoring.
- Customizable Naming .e.g. Remote monitor
- Verify Forms or individual data points
- Permission based verifications
Lock data when quality assurance has been performed to secure data for analysis.
- Lock individual data events
- Database locking
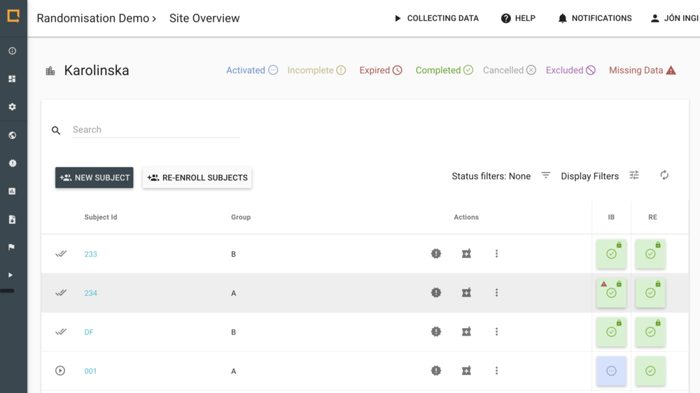
Lock data when quality assurance has been performed to secure data for analysis.
- Lock individual data events
- Database locking
Get a quick overview of your Subject population, export data when needed and get a complete overview of site compliance and subject response rates. Supports statistical tools such as SPSS, SAS, STATA, R, Matlab, MS Excel and more.
- Export data by site or whole study
- GDPR compliant export
- Custom export labels
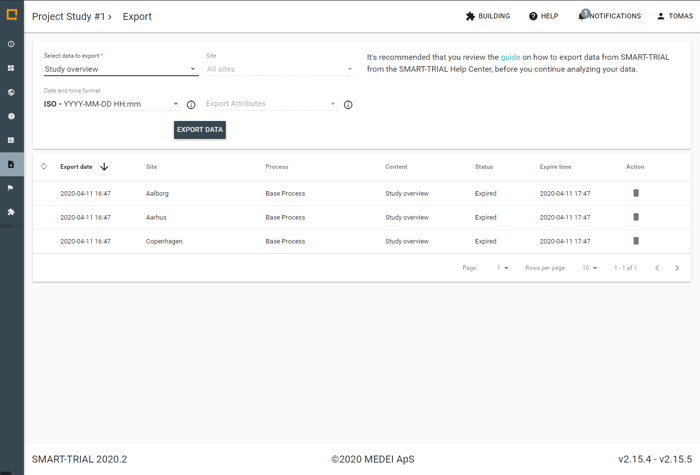
Get a quick overview of your Subject population, export data when needed and get a complete overview of site compliance and subject response rates. Supports statistical tools such as SPSS, SAS, STATA, R, Matlab, MS Excel and more.
- Export data by site or whole study
- GDPR compliant export
- Custom export labels
Enable SMS send-oust for quick responses from subjects and increase your response rates.

Enable SMS send-oust for quick responses from subjects and increase your response rates.





Simplifying MedTech Regulatory Compliance
Designed and developed in compliance with the PIC/S Guidance, PI-011-3 Good Practices for Computerized Systems in Regulated “GxP” Environments, with software validation based on IEC 62304.
We ensure that you can be compliant with the industry latest standard on GCP for Medical Devices
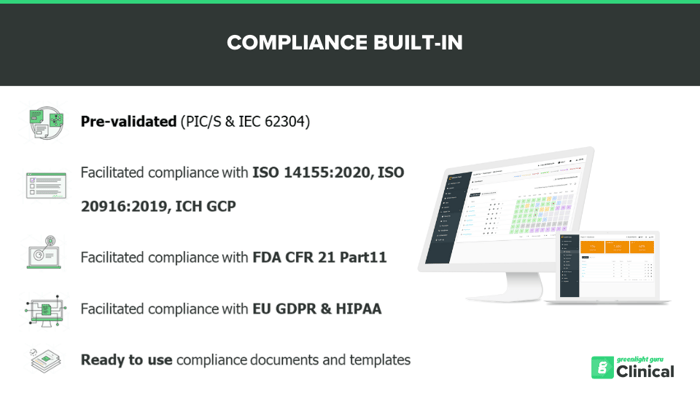
We ensure that you can be compliant with the industry latest standard on GCP for Medical Devices
We deliver ready-to-use SOP templates you can customize to your own QMS or study protocol. Streamline the documentation process and compliance with our SOP templates and validation templates.

We deliver ready-to-use SOP templates you can customize to your own QMS or study protocol. Streamline the documentation process and compliance with our SOP templates and validation templates.
Greenlight Guru Clinical is designed to protect your data by following industry-leading standards on security, encryption, and access control. The platform comes out-of-the-box with two-step verification, permission-based access control, and extensive system audit logging, all compliant with regulatory requirements.

Greenlight Guru Clinical is designed to protect your data by following industry-leading standards on security, encryption, and access control. The platform comes out-of-the-box with two-step verification, permission-based access control, and extensive system audit logging, all compliant with regulatory requirements.
Greenlight Guru Clinical simplifies regulatory compliance for ISO 14155 (GCP), FDA 21 CFR Part 11, GDPR, and HIPAA by offering ready-to-use QA templates, system modules, and guidance documents.
Read the GCP, FDA Part 11, and HIPAA Compliance Statement

Greenlight Guru Clinical simplifies regulatory compliance for ISO 14155 (GCP), FDA 21 CFR Part 11, GDPR, and HIPAA by offering ready-to-use QA templates, system modules, and guidance documents.
Read the GCP, FDA Part 11, and HIPAA Compliance Statement




MedTech Expert Services
Greenlight Guru Clinical experts also offer consultancy, training and custom development services.
Our data management experts can take care of your study setup in Greenlight Guru Clinical, including all forms, questionnaires, validation rules, process design, and ePRO templates for e-mails and SMS.

Our data management experts can take care of your study setup in Greenlight Guru Clinical, including all forms, questionnaires, validation rules, process design, and ePRO templates for e-mails and SMS.
We can deliver both online and on-site training for your sites or end-users. This ensures that all users, investigators, monitors, and other study staff will be comfortable in using Greenlight Guru Clinical thereby increasing compliance and data quality as well.
We can deliver both online and on-site training for your sites or end-users. This ensures that all users, investigators, monitors, and other study staff will be comfortable in using Greenlight Guru Clinical thereby increasing compliance and data quality as well.
With over a decade of experience in medical software development our team can implement custom features or integrations to Greenlight Guru Clinical as needed.

With over a decade of experience in medical software development our team can implement custom features or integrations to Greenlight Guru Clinical as needed.













