top takeaways
.png?width=65&name=1%20(2).png)
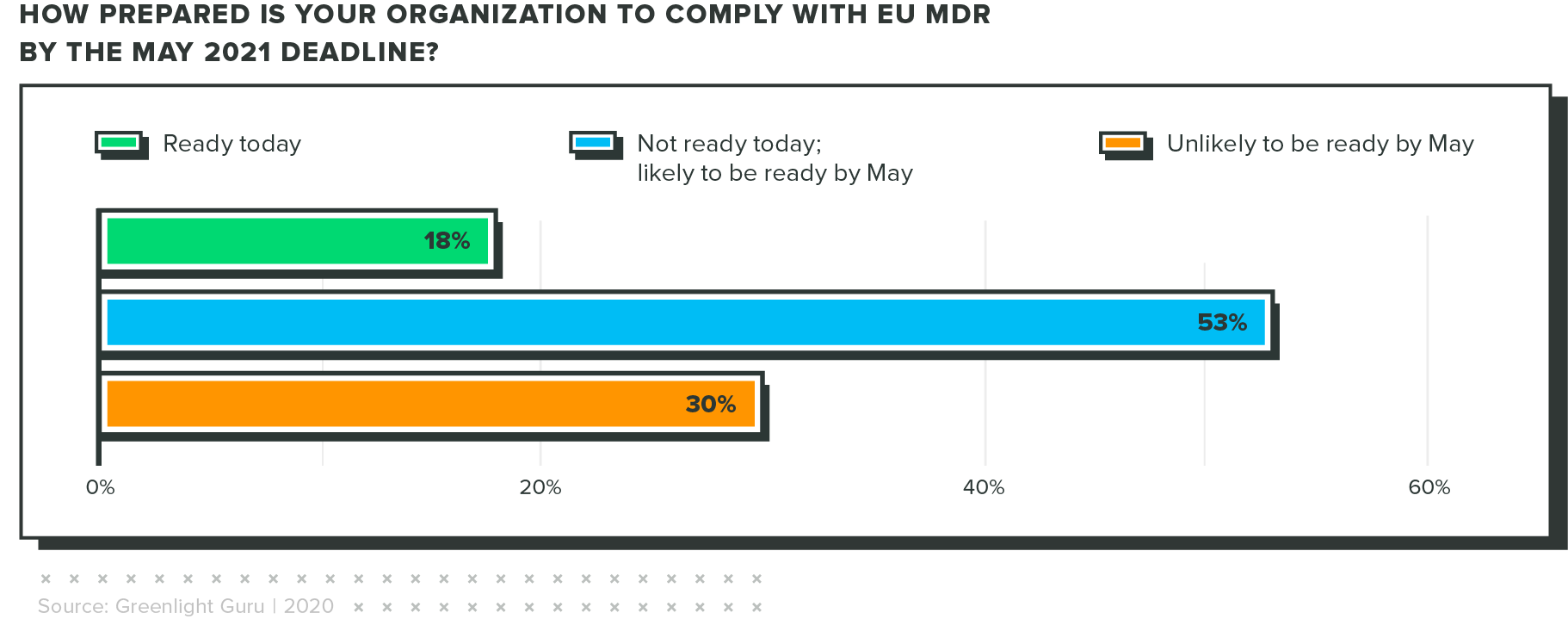
Only 18% are ready for eu mdr today
.png?width=65&name=2%20(1).png)
Obstacles are related to knowledge, clarity, and resources

There are many points of friction hiding in plain sight
Preparing for the May 2021 EU MDR Deadline
When COVID hit, the EU MDR implementation deadline was bumped back a year. Industry regulators hoped this delay would make it easier for companies to comply with the new regulations. Unfortunately, the results of our survey show that many respondents won't be ready by the new deadline.
This puts quality and regulatory pros in a tough position. There is a lot of work to be done to comply with EU MDR, but as of this report, there's very little time to catch up if you're behind.
Some who will not meet the May deadline are counting on a reprieve due to registering their products under MDD. These companies must comply with EU MDR by the expiration date of each CE mark rather than by May 2021. They would be unable, however, to register any new products without current EU MDR compliance.
In this report, we wanted to provide you with a holistic picture of what other medical device teams are doing to prepare for the upcoming deadline and give you a full 60 day action plan to get prepared, regardless of your current state of preparedness.
Get your free copy of
2021 EU MDR Preparedness Study Report
%202021%20EU%20MDR%20report.png?width=390&name=(cover)%202021%20EU%20MDR%20report.png)
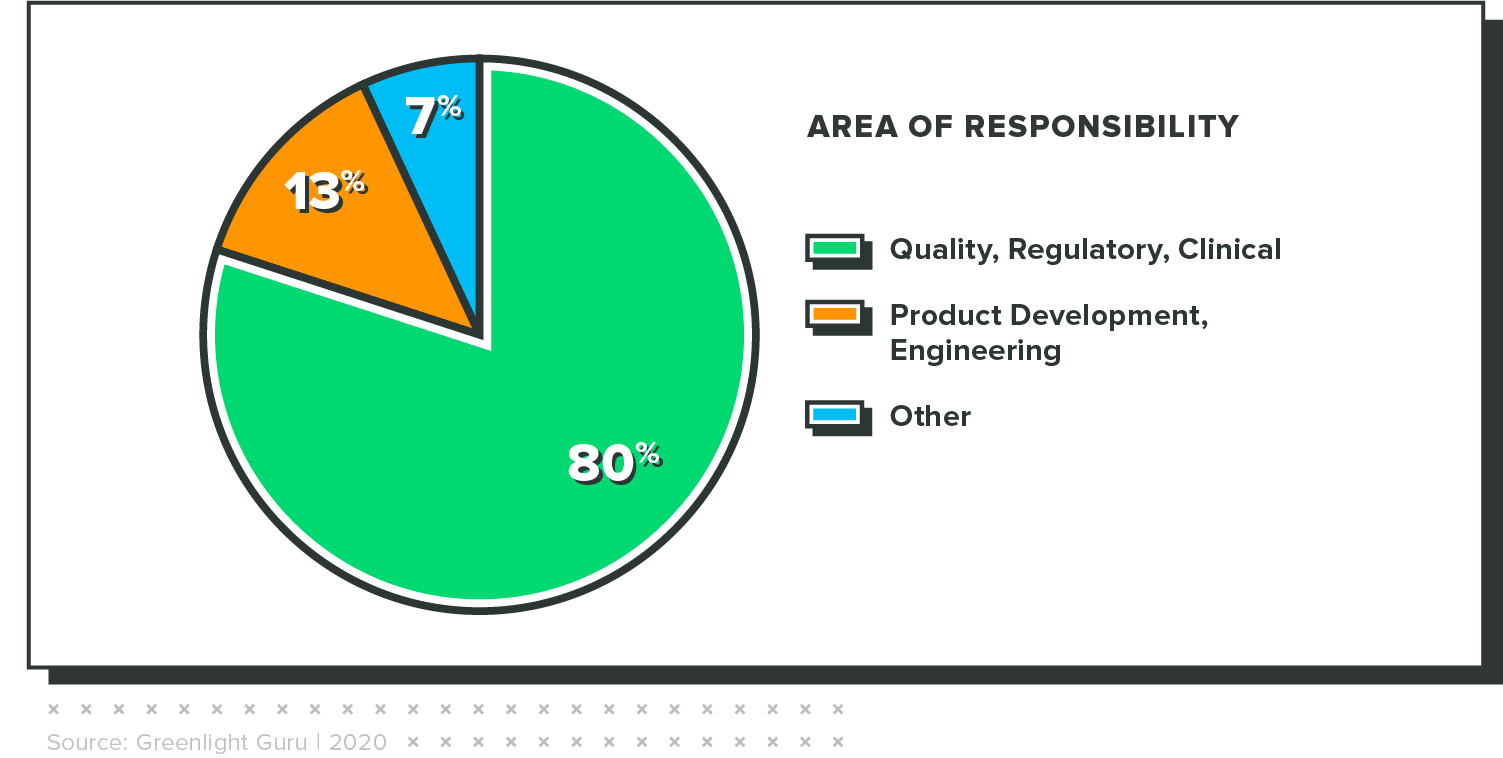
WHO TOOK THE SURVEY
The results in this report are from an online survey that was fielded from January 24 through February 8, 2021. It had 230 respondents, all of whom completed the survey. Key demographic variables are included below.
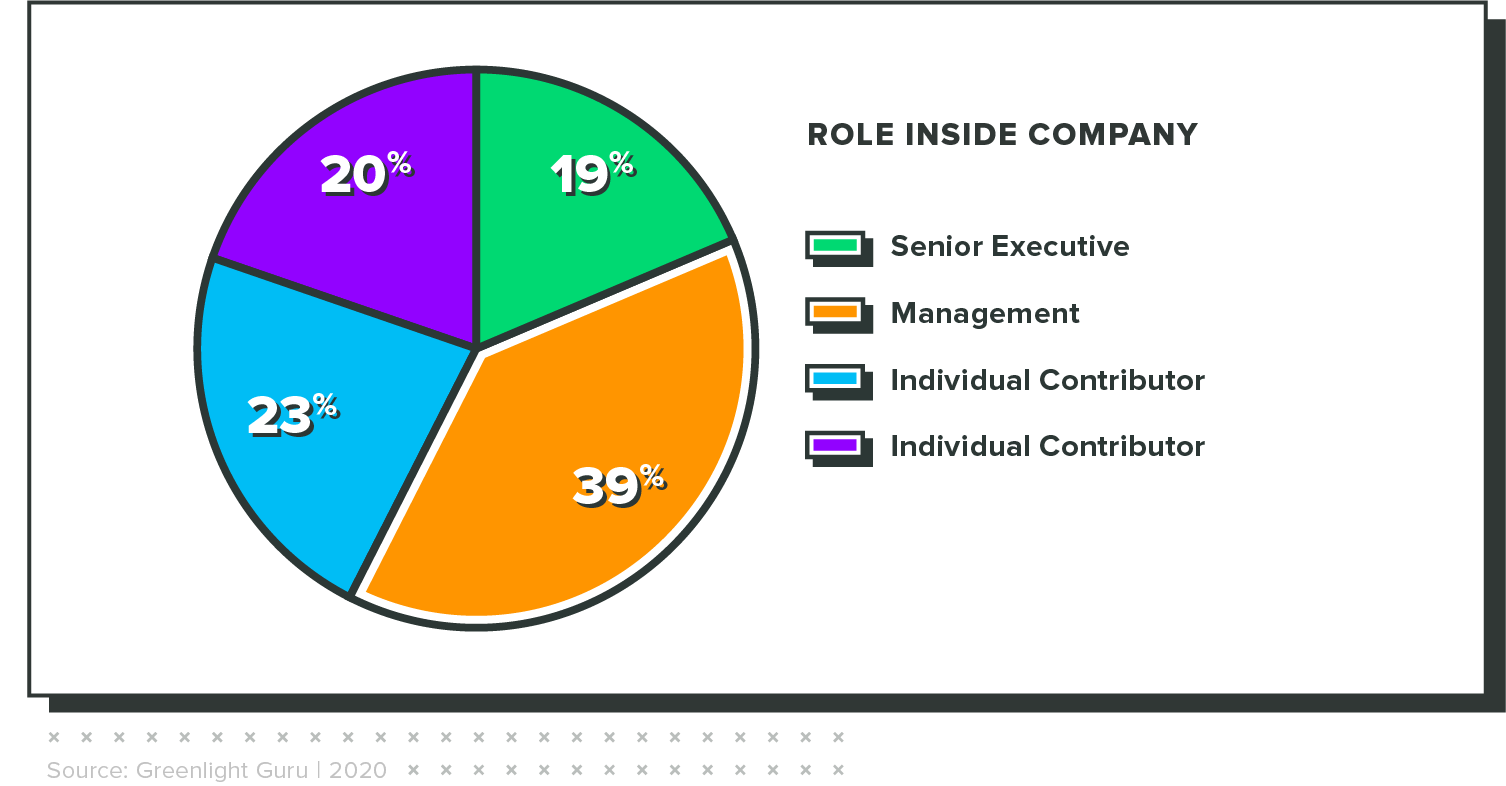
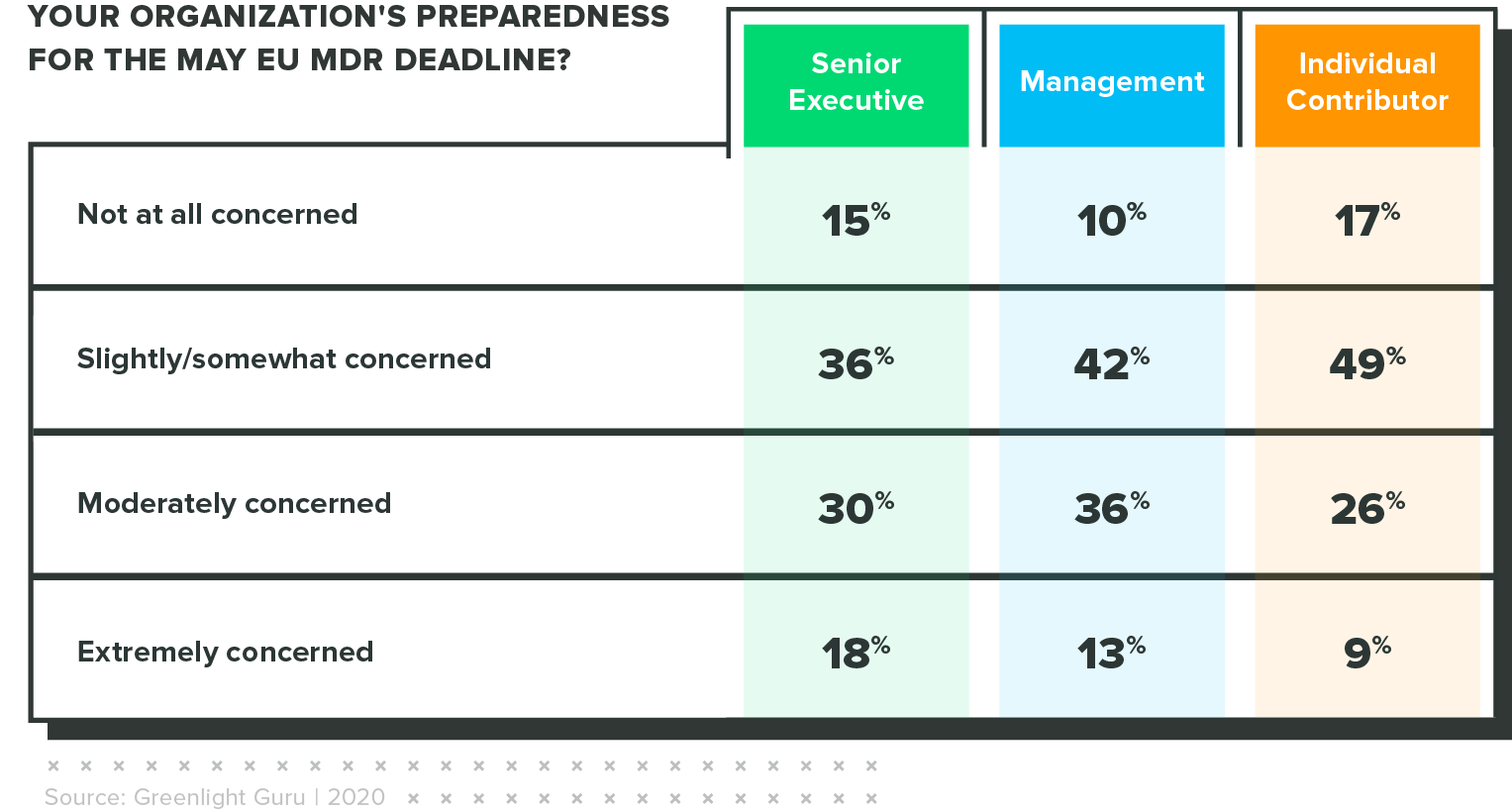
Concern is highest among executives
When rating their level of concern regarding this lack of preparedness, senior executives were 2x more likely than individual contributors to say they were "extremely concerned" about the approaching deadline.
This could signal the significance of the business risk associated with non-compliance.
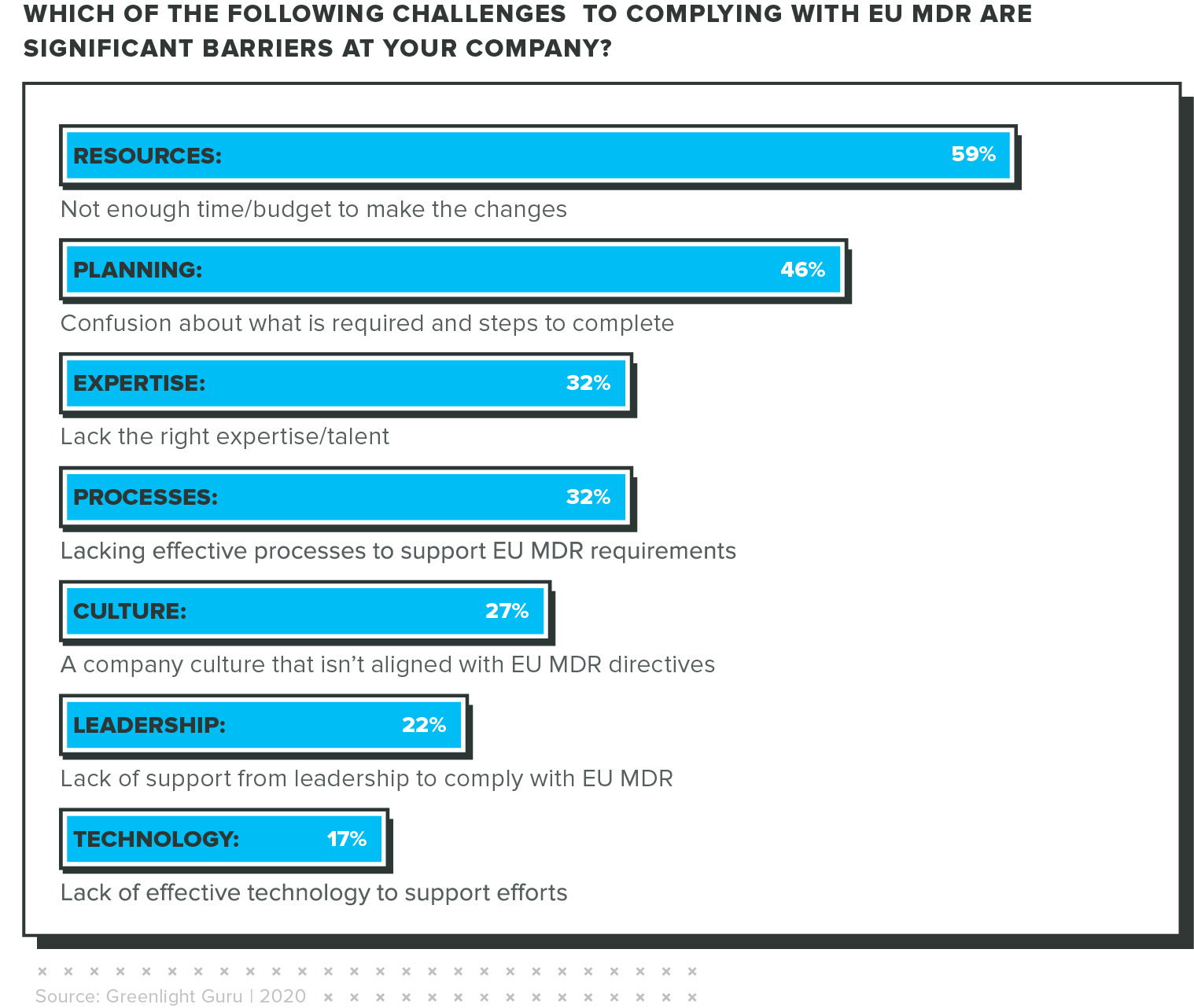
The Obstacles We're Facing
Respondents identified several obstacles that are preventing them from achieving EU MDR compliance today.
Unlike our findings from our 2021 State of Medical Device report, we saw that leadership and culture weren't strong contributing factors to this lack of preparedness. Instead, respondents were more concerned about resources, processes, and clarity.
Hidden points of friction
Beyond the critical action items, the survey also aimed to uncover hidden areas of challenge and friction — specifically, where different stakeholders within medical device companies disagree about key issues.
|
|
70% have not developed an operational plan for business continuity |
|
|
62% have not determined a reclassification plan |
|
|
51% haven't documented a strategy to remediate gaps in clinical data |
|
|
38% said they are currently unable to incorporate PMS data into their technical documentation |
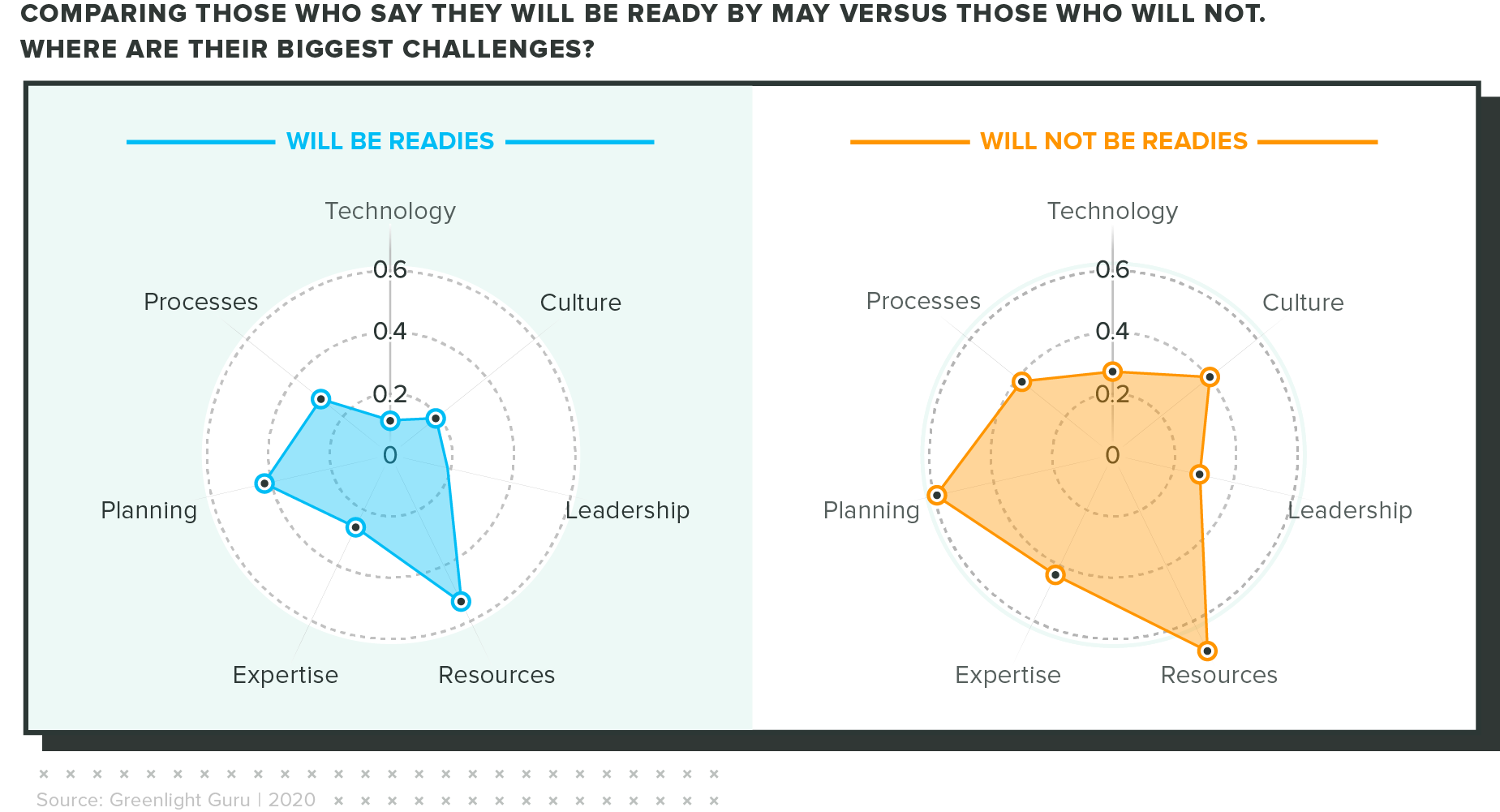
What sets aside those who are prepared?
We looked at the difference between companies who said they will be ready by May 2021 and those who said they won't. Ultimately, the factors that are holding them back are similar, but the "won't be readies" saw big gaps in resources and planning necessary to hit the deadline.
What can you do to prepare for the deadline?
DOWNLOAD THE FULL REPORT TO UNCOVER a 60-day action plan for EU MDR compliance.

%202021%20EU%20MDR%20report.png?width=2400&name=(cover)%202021%20EU%20MDR%20report.png)
-2.png?width=500&height=501&name=GG-LinkedIn-profile-pic-green-1%20(1)-2.png)
.png?width=232&name=Report%20Download%20CTA%20(1).png)


