Class I-III? We’re Here To Help.
No matter your classification, everything begins with an idea. Whether you plan on submissions with the FDA or EU MDR, you need an end-to-end QMS solution designed specifically for MedTech. Don’t settle for less.
 Calculate Your ROI
Calculate Your ROI
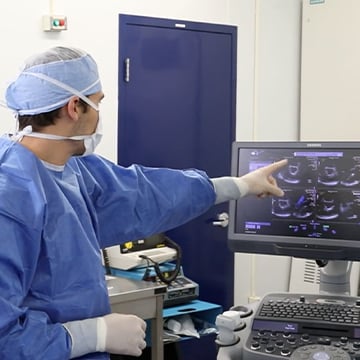



This Work Is Hard. We’ll Make Your Job Easier.
Starting off with the right foundation to develop high-quality devices is challenging. It’s easy to lose sight of creating a culture that values excellence and quality when you’re juggling funding, regulatory, design, product development, laboratory procedures, growing markets, competitors, and rapidly growing technology. All these areas can become roadblocks if not managed properly in an end-to-end system.
Your Device
Classification Matters
Whether you are submitting to the EU, FDA, Health Canada, or others, your path to market and steps to success are determined by your classification and requirements that follow. Getting started with a world-class QMS tailored to your device type is the best way to align with regulatory guidelines.

Class I
Whether you plan on registering with the FDA, getting technical documentation prepared, or applying for a medical device license, an end-to-end QMS platform will help you turn quality processes into an asset to develop safer, more effective products.
-1500x908-01.png?width=750&name=501(k)-1500x908-01.png)
Class II
510(k) submissions, notified bodies, technical documentation, and ISO 13485 can become frustrating and overwhelming to manage. Achieving compliance can ultimately decide the fate of your device and company as a whole. Start off on the right foot with an end-to-end QMS that is also a platform for accelerating product development.

Class III
Reduce your risks with a platform that’s aligned with the latest industry standards for Class III medical devices. ISO 13485, 510(k), premarket approval (PMA), CE marking, and undergoing continuing audits will be critical to your success. Keeping the design and development momentum going at the same time as maintaining compliance can be challenging. Leverage a platform that has compliance built-in, so you can focus more on developing high-quality products, instead of compliance paperwork.
It Begins With What You Know
75% of medical device market leaders provide their employees with educational opportunities. Access the industry knowledge you and your teams need to excel. Join a community of Greenlight Guru users all striving towards a common goal of improving the quality of life.

Class I
Whether you plan on registering with the FDA, getting technical documentation prepared, or applying for a medical device license, an end-to-end QMS platform will help you turn quality processes into an asset to develop safer, more effective products.
-1500x908-01.png?width=750&name=501(k)-1500x908-01.png)
Class II
510(k) submissions, notified bodies, technical documentation, and ISO 13485 can become frustrating and overwhelming to manage. Achieving compliance can ultimately decide the fate of your device and company as a whole. Start off on the right foot with an end-to-end QMS that is also a platform for accelerating product development.

Class III
Reduce your risks with a platform that’s aligned with the latest industry standards for Class III medical devices. ISO 13485, 510(k), premarket approval (PMA), CE marking, and undergoing continuing audits will be critical to your success. Keeping the design and development momentum going at the same time as maintaining compliance can be challenging. Leverage a platform that has compliance built-in, so you can focus more on developing high-quality products, instead of compliance paperwork.

It Begins With What You Know
75% of medical device market leaders provide their employees with educational opportunities. Access the industry knowledge you and your teams need to excel. Join a community of Greenlight Guru users all striving towards a common goal of improving the quality of life.



















