When I first learned about the FDA’s Case for Quality initiative, I immediately wanted to learn more.
Why?
It’s been my observation in my career in the medical device industry that there is somewhat of a contentious relationship between FDA and industry. Medical device companies do not view FDA as a collaborative partner. Instead, FDA has historically been viewed as a gatekeeper and a policing agency. The focus seems to be that of addressing compliance to the regulations.
And this has bothered me. Is compliance the objective? Or is there a bigger objective?
I believe there is a bigger objective. An objective that focuses on patients and improving quality of lives. And the FDA Case for Quality is a program designed to help shift from being just compliance oriented to what truly matters: true quality.
Once I had a better understanding about the FDA Case for Quality program, I knew it was imperative for Greenlight Guru to partner with FDA to help spread the word about this program. And we are doing just that through a multi-part webinar series with Cisco Vicenty, FDA CDRH Program Manager - Case for Quality. In fact you can check out the Case for Quality overview and status update webinars. Be sure to also sign up for the third installment which will include a summary of the pilot program to date.
The FDA CDRH vision is about ensuring patients in U.S. have access to high-quality, safe, effective medical devices. All about the patient and driving best health outcomes possible.
By being compliance focused, however, it seems as though what is best for patients can easily get lost.
FDA has been observing several industry behaviors.
- High industry focus on regulatory compliance instead of adopting best quality practices
- Manufacturers not adopting automation and digital technologies
- Little to no competitive market around medical device quality
These observations are what has prompted the need for Case for Quality.
FDA Case for Quality Pilot Summary, Learnings, and Next Steps. Watch for Free →

The Case for Quality pilot is a voluntary program taking place throughout 2018. Participation includes a capability maturity model integration framework to assess company’s ability to produce high-quality devices with ability to increase patient safety. Medical device companies participating in the pilot will forgo surveillance, post-approval, and risk-based FDA inspections. Additionally, participants benefit from streamlined submissions for manufacturing change notices, site changes, and PMA notifications.
The big idea behind the Case for Quality pilot is to establish initiatives in collaboration with industry to shift away from policing, compliance initiatives and ultimately improve patient outcomes. It is about enabling and encouraging partnerships between FDA and medical device companies. Case for Quality is about driving better integrations within a company’s business systems and processes for how and why the work is being done in order to drive desired outcomes and results.
From Compliance to Operational Excellence![From Compliance-Operational Excellence-3]()
In addition to improving patient outcomes by focusing on true quality, there are additional benefits for both FDA and industry regarding resource constraints on both sides. FDA benefits from reduced load on CDRH resources and FTEs required for inspections and reviews of submissions. Medical device companies benefit from improving product and process quality.
Companies also benefit from reduced internal resource strain currently required to prepare and support submissions and compliance-based inspections. All of this is for the purpose of getting products to the patients who can benefit sooner. Another side benefit is that reducing time to market for companies will reduce operational expenses and result in revenue realization sooner.
As an example, FDA has seen real benefits with company collaboration already, even in the pilot phase, as it relates to streamlining PMA 30-day notices. Under the current compliance-based model, there were 30-day notices that might not have been pursued due to resource costs and constraints.
With the Case for Quality model, these 30-day notices were able to be streamlined, reviewed, and approved within a much shorter timeframe while reducing resource strains for both FDA and industry. As a result, there has been a direct patient benefit.

The current regulatory focus revolves primarily around a company's quality system. Running a medical device business requires more than just this, especially when evaluating how medical device companies operate. The need is to have a more holistic view of what happens within an organization.
How are tools and resources implemented? What are the company’s values and principles? What are the company’s capabilities and how do these align with their culture, behaviors, and results?
Creating a Virtuous Cycle of Improvement

How is the Case for Quality model truly different from the traditional way FDA assesses medical device companies?
The first step is to understand the capabilities of the medical device company. This involves a series of interviews to understand the business, involving process stakeholders—not just those who handle audits and inspections. These interviews are not an interrogation or typical compliance-based inspection.
The purpose of the interviews is to identify areas for improvement for the medical device company. It’s intended to help enable a company’s ability to be more proactive, rather than just reacting to correct problems.
The model leverages the Capability Maturity Model Integration (CMMI) appraisal. CMMI is not a quality system or a standard. It is a set of best practices that determine a business framework and baseline to assess and evaluate capabilities to more easily identify opportunities for improvement. The CMMI model assesses strengths and QMS framework established by the company. Part of the objective is to assess how well is QMS being executed to get the work done.
Note that FDA QSR compliance is a prerequisite for inclusion in the pilot. But involvement in the pilot allows a company to forego routine compliance inspections while being part of the Case for Quality initiative..
The Case for Quality initiative has also been driving improvements in FDA processes as well. Some examples include reducing manufacturing change notice reviews from 30 days down to 5 days, manufacturing site change reviews down to 1 week target, and improvements to PMA processes.
As a result, industry is a direct beneficiary because the early results from the program as showing a reduction in FDA review times related to key manufacturing related items, as well as reducing the burden and disruptions of inspections.
Further opportunities FDA envisions with Case for Quality include ability to help accelerate review and approvals required for product changes, leverage the methodology more for design and development—including inclusion of 510(k) reviews, and freeing up resources to focus on continuous process improvement and innovation.
How is a Maturity Appraisal Different?
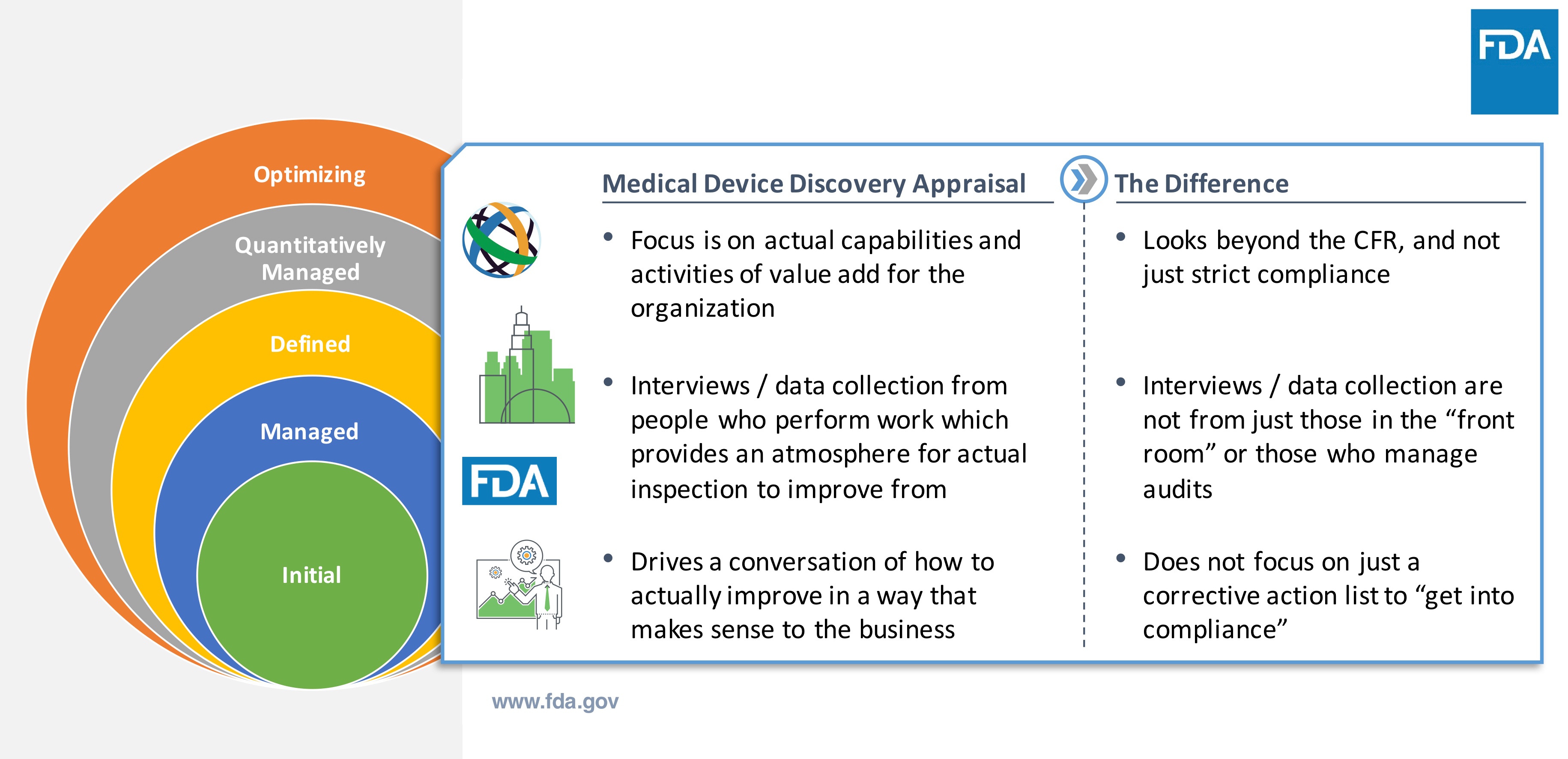
How does a medical device company participate with the Case for Quality? There is an application process that includes CMMI Institute, a third party resource to help provide objectivity and to perform the appraisal assessments. After acceptance, appraisal team starts working with company.
This involves a deep dive into the existing processes at the medical device company to understand current state and desired outcomes. From there, it’s about collaborating and learning to drive quality initiatives.
Note that there is no need to do extra preparation to engage in the process. It’s actually quite the opposite since the first steps are about discovery and having conversations to understand current state of the company. This helps define the existing baseline for the company going through the Case for Quality. Reiterating, this engagement is not an audit, it’s not a FDA inspection.
Voluntary Pilot Overview

After completing the initial appraisal assessment, the medical device company is provided a detailed process capability report to identify strengths and weaknesses. This is very granular and specific to their business and is prepared by CMMI and visible to the company and not FDA.
Rather, a high-level overview is provided to FDA in order to establish the baseline of company practices. Recall that FDA already knows that compliance criteria has been addressed because this was part of the inclusion criteria into the pilot program.
Information Collected
The appraisal assessment is a means to help identify better systems and approaches to identify opportunities for product and process improvements. The methodology is about being proactive versus reactive and to address real problems immediately.
The premise of the entire Case for Quality initiative is to simplifying FDA review processes. It is a means to embrace agency / industry collaboration and provide a format and conduit for meaningful information exchange with the desired purpose for better patient outcomes. The Case for Quality program aligns with CDRH vision. Frankly, it aligns with the mission’s of many medical device companies—improving quality of life.
Yes, there is a fear that some companies have raised about this model and approach. Fear that FDA will have access to and use this data and information to identify compliance observations and to provide evidence for downstream inspections. However, FDA’s overall intent is eliminate defensive nature of a traditional compliance-based FDA inspection and improve collaboration to improve patient outcomes.
It has been stated by FDA CDRH, including from Dr. Shuren, there is an interest to remove the term “compliance” from FDA’s lexicon over the next few years. Compliance and enforcement have been synonymous for far too long. Driving focus on quality is about what is best for patient. Compliance should not be the go to approach / tool to do this.
Those participating in the pilot study to date have indicated that the quality focus is welcome and that compliance is still being addressed in a healthy manner.
Keep in mind that this Case for Quality approach is new to the medical device industry. And with that, there are some perceptions and assumptions of traditional compliance-based FDA inspection applying to Case for Quality CMMI appraisal. Know that these are different approaches.
With Case for Quality, appraisers have discussions with the actual people who are doing the work within the organization. It’s not about collecting documents and records. It’s about assessing process capabilities. It’s about having real, engaging conversations versus just answering questions.
How are Manufacturers Perceiving the Difference in the 2 Processes?

Part of the result of this process is participating companies receive very detailed, granular data and information about their current products and processes. This is key to understanding current state and identifying opportunities for improvement.
FDA receives high-level overviews of each pilot participant, allowing a baseline of quality to be established for the company. This provides the agency more insights into trends and patterns into specific areas relating to quality systems effectiveness and current compliance initiatives.
Additionally, this streamlined approach is very beneficial to FDA to better utilize resources and to simplify change notifications and reviews. The Case for Quality provides a framework to better analyze data, drive improved quality metrics, identify areas for more efficient resource allocation, and understand industry needs.
For example, are industry and FDA paying attention to the same things? The Case for Quality hopes to achieve better definition of objective metrics to allow best possible insights as to when to take action and better alignment between what companies use to evaluate business continuity and quality, while aligning with FDA.
Improved quality metrics are a desired outcome of the pilot program. Historically, metrics used by both medical device companies and FDA are more traditional compliance-based indicators versus quality-based metrics. And this is one area of the Case for Quality that has been more challenging to make the shift from compliance oriented to shifting to true quality.
FDA Case for Quality Pilot Summary, Learnings, and Next Steps. Watch for Free →
Compliance Indicators VS. Quality Indicators

While there are cases for the traditional compliance indicators, shifting the focus to quality-based metrics will have a profound impact on patient outcomes.
For example, having metrics for product safety, effectiveness, reliability, and availability are means to put quality KPIs front and center. A goal is to shift to quality-based metrics within a company to align with product and process needs that have highest impact on addressing patient needs and outcomes.
And ultimately, the goal is to drive alignment with FDA and the company—so that both are speaking the same language with the same overall objective with focus on patients. In order for this shift to be possible, it is necessary to have transparency and effective communication between industry and the agency to achieve this alignment.
Jon Speer is a medical device expert with over 20 years of industry experience. Jon knows the best medical device companies in the world use quality as an accelerator. That's why he created Greenlight Guru to help companies move beyond compliance to True Quality.