The Biggest Quality Challenges for Medical Device Companies in 2025

One of the major themes in the 2025 Medical Device Industry Report is that medical device companies face considerable challenges in building and improving their quality processes.
Quality has long been a concern in the industry—first and foremost because it affects the safety and effectiveness of the devices that patients rely on. But from a business standpoint, quality is the key to both making great devices and staying compliant with regulations while doing so.
In this article, we’ll take a look at some of the insights from the 2025 Medical Device Industry Report regarding quality departments, and how medical device companies are working to overcome challenges with quality.
BONUS RESOURCE: Click here to download the full 2025 Medical Device Industry Report for free!
What quality challenges are medical device companies facing in 2025?
The 2025 Medical Device Industry Report surveyed more than 500 medical device professionals, and two-thirds of the respondents work in quality and regulatory—providing an inside look into the world of QA/RA. And respondents at both pre-commercial and commercialized companies told us that implementing or upgrading their quality system processes was a top objective.
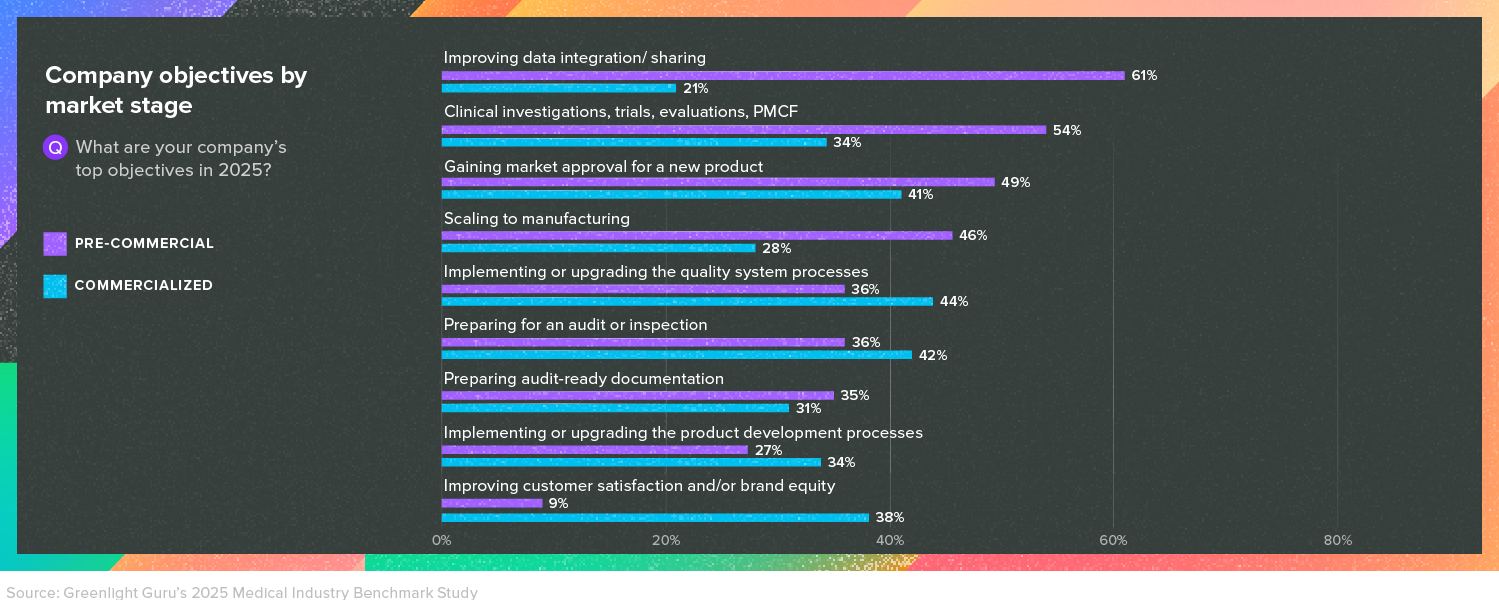
Getting quality systems up-to-date is a key objective because the QMS requirements in regulations like EU MDR and the QSR in the US are non-negotiable. And while they are legal requirements, they’re also subject to change. Evolving regulations in both the EU and the US are forcing medical device companies to reassess their quality programs.
For instance, when we asked respondents what they believed their company was still unprepared for with regards to EU MDR, nearly half of both pre-commercial and commercialized companies cited keeping up with the additional QMS requirements.
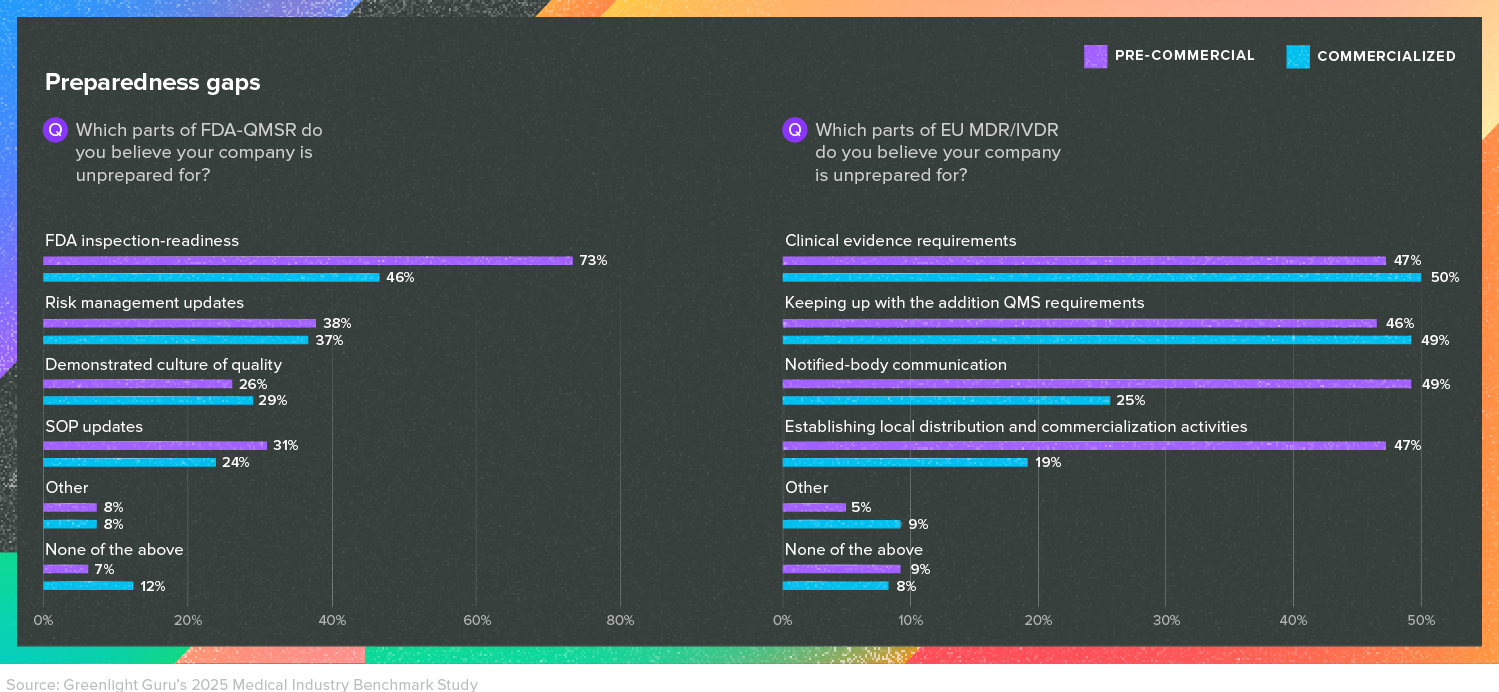
And for companies marketing in the US, many businesses are worried about their preparedness for an FDA inspection under the new Quality Management System Regulation (QMSR), which will go into effect in February of 2026. Though the changes associated with the QMSR are far less dramatic than those of EU MDR, the FDA is unlikely to extend the deadline for compliance.
Internal silos exacerbate struggles with quality
While quality assurance is often considered the Quality department’s domain, it should really be everyone’s concern. Quality events like nonconformances and CAPAs often involve several different departments, which means getting to the bottom of a problem and implementing a solution will require cross-functional collaboration.
However, respondents to the 2025 Medical Device Industry Report told us that internal silos are a major issue for medical device companies—especially as they grow larger.
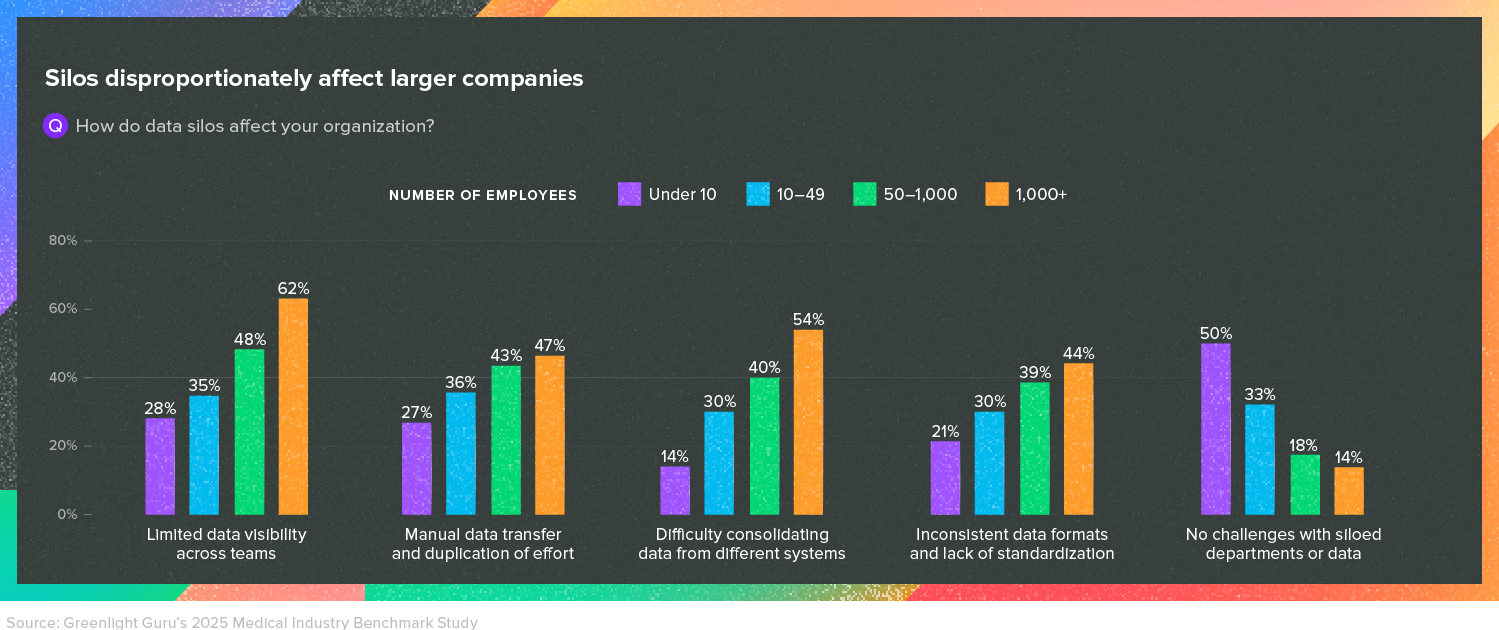
In fact, Vincent Cafiso made this point when he came on the Global Medical Device Podcast recently. Over his 30-year career, Vincent has noticed that smaller companies naturally have a lot of cross-functional collaboration because everyone knows each other and they may even be working in close physical proximity.
But as departments grow, walls start to emerge between them. It’s much more difficult to get a hold of someone in another department and collaborate with them.
Perhaps that’s why our survey also found that as companies grow larger, the number of hours quality employees spend on reactive remediation activities tends to balloon—from 16 hours per month for companies with under 10 employees to 76 hours per month for companies with more than 1,000 employees.
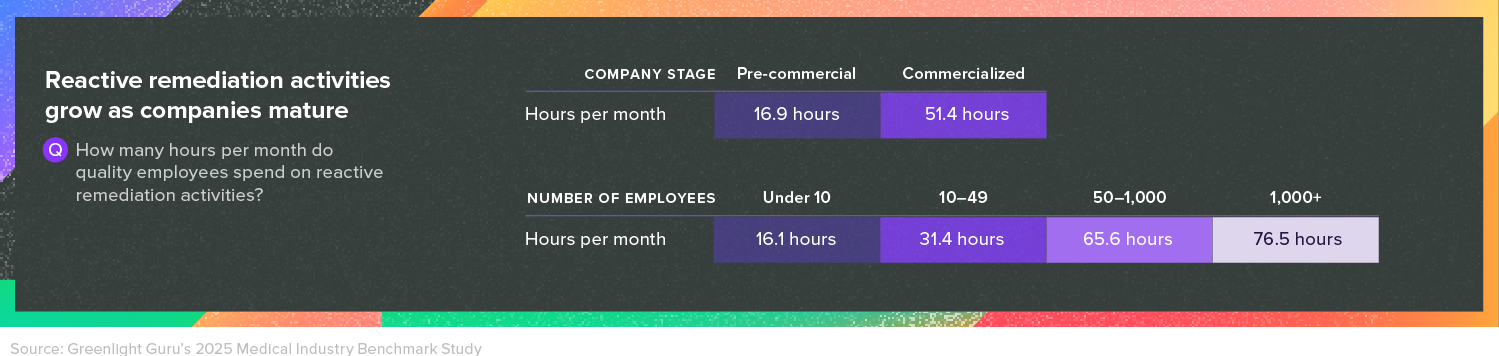
The only way to break down those walls and encourage collaboration across departments is to make a deliberate effort to do so. The path of least resistance is always to keep talking to people you know and trying to solve problems on your own. But as Vincent says, “At the end of the day, you’re all there to either improve the existing products or bring out new innovative products, and it can’t be done in a silo.”
One final stat to keep in mind here—respondents that told us they were very well equipped to reach their quality objectives this year were also six times more likely to say their organization is highly collaborative (as opposed to highly siloed).
Modernizing the medical device tech stack is key to solving quality challenges
The medical device industry, with its unique regulatory requirements and standards, is tough to navigate with spreadsheets or general-purpose software that’s built to be used by pharma, aerospace, or automotive companies. General-purpose tools typically require lengthy validation and implementation and need to be heavily customized for medical device companies.
In fact, our survey found that companies using industry-specific tools for quality management—that is, solutions designed for the medical device industry—were twice as likely to say they were equipped to meet their quality goals than those using general-purpose tools.
Companies using general-purpose tools primarily cited budget constraints and fears about implementation and integration into an existing tech stack. And yet, 69% of respondents told us they were not “very confident” their current quality management system can handle their company’s projected growth over the next 12 months.
As Greenlight Guru’s own Etienne Nichols points out, the dissonance between those two ideas—we need a new QMS and a new QMS will be too expensive—can be resolved if companies take the long view.
“The truth is that the cost of delaying technology investments can end up costing even more in the long run. The problem is less visible in the earliest stages of development, but as a company ramps toward launch, the sheer volume of data can quickly overwhelm teams and cause a tremendous amount of inefficiency.”
BONUS RESOURCE: Click here to download the full 2025 Medical Device Industry Report for free!
Turn quality into a competitive advantage with Greenlight Guru
With so much riding on your quality processes, you can’t afford to use outdated general-purpose software or paper-based systems to manage your QMS.
With Greenlight Guru, you’ll get modern software solutions built specifically for medical device companies like yours, which means you’ll have everything you need to turn quality challenges into competitive advantages. Our software comes aligned with the requirements of 21 CFR Part 820, ISO 13485:2016, ISO 14071:2019 and EU MDR. You’ll also have access to more that 80 SOP templates and guidance from our in-house MedTech experts. In short, our QMS software gives you everything you need to build a compliant and organized quality system.
Ready to see how a purpose-built QMS solution can take the uncertainty out of compliance? Then get your free demo of Greenlight Guru today!
Matt McFarlane is the Senior Content Writer at Greenlight Guru. He is an avid reader and writer, specializing in the medical device industry and its many regulations, standards, and guidance documents.
Related Posts
Insights from an Ex-FDA Investigator: Compliance, Quality Systems, and MedTech Trends
2025 Medical Device Industry Report: Quality Challenges, Regulatory Complexity, and Economic Uncertainty
FDA Publishes Final Rule on QMSR
Get your free report
2025 Medical Device Industry Report










