Introducing Document Comparison: Boost Audit Readiness, Accelerate Training, and Streamline Approvals

There may be hundreds—even thousands—of documents living in the quality management system (QMS) of a medical device company. And many, if not most, of those documents will need to be changed over their lifetime. There are endless reasons to change a document, such as a standard operating procedure (SOP) that needs to be updated to reflect a new method for assembling a device.
Ensuring the correct changes happen and that everyone is working with the same document is essential to building safe and effective medical devices. But just having the latest version of a document isn’t the only thing that matters. You also need to be able to see what has changed within the document and how it may affect related processes—quickly and with minimal effort on the part of your team.
Manual document comparison is a productivity killer
Manual document comparison is enormously time-consuming and prone to error. Asking someone to spot the differences between two versions of a ten-page document is simply not an efficient way to operate. And the drag on productivity has knock-on effects across your business.
-
Manual document comparison forces employees to leave the QMS software to manually compare documents side-by-side. Not only is it inefficient, it also requires individuals to always spot every change.
-
Training slows down, especially when employees are being retrained on lengthy documents with small changes to them. When training managers and employees don’t know what’s been changed on a document, they have to search out what may be minor changes, slowing the training process and keeping your team from doing meaningful work.
-
Audit prep can be tricky without a clear understanding of what’s been changed in the most recent version of a document. Without clear version tracking, it may be unclear why a document was updated or if the changes in documentation have been implemented across the business.
Document Comparison: see the difference between your documents
Greenlight Guru has added a Document Comparison feature to our QMS software, allowing you to quickly and clearly see exactly what’s been changed between versions of a document—without leaving the system. Now, all the changes you make to a document within Greenlight Guru can be tracked and highlighted for users, so even infrequent users of the QMS will understand what’s changed at a glance.
With Document Comparison:
-
There’s no need to leave the system to compare documents side-by-side. Changes are highlighted and clearly visible.
-
Reviews and approvals are faster with changes clearly visible for the approver.
-
Training is faster and less burdensome, as the difference between old and new versions of SOPs and other documents are highlighted for users.
-
Demonstrating what’s changed and why during audits is now much simpler with the comparison feature.
This is more than just “redlining.” This is the quick and easy comparison of documents you’ve been asking for, and it happens right inside the system you use for everything else.
Want to see it in action?
Here’s a peek at what Document Comparison looks like within Greenlight Guru’s QMS.
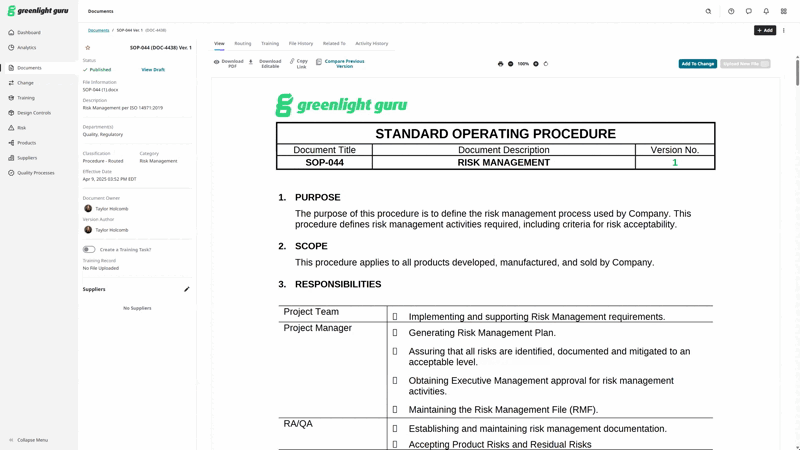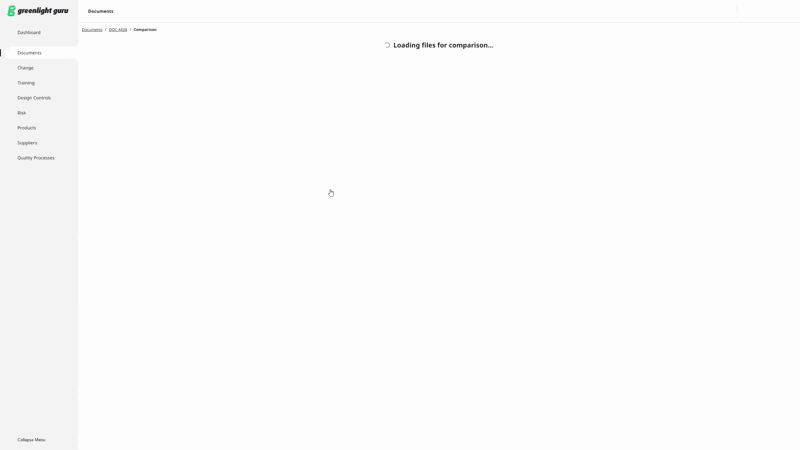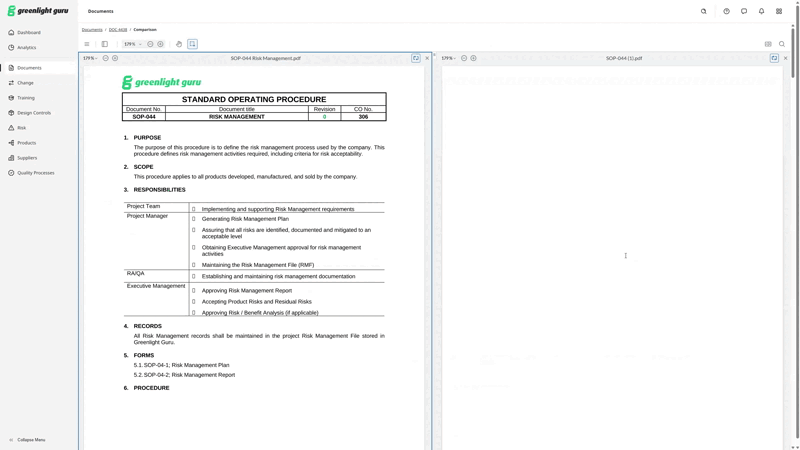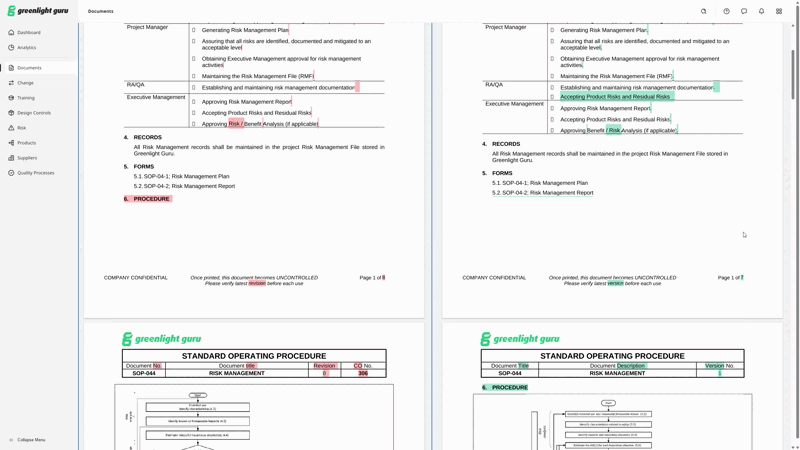
Create, find, and manage all your medical device documentation with Greenlight Guru
Document management is the heartbeat of a great medical device company. Your documentation and records describe everything about your devices and the work you’ve done to ensure they’re safe and effective for the patients who rely on them. Managing all that documentation and ensuring it’s up-to-date and correct is critical to your ability to deliver on your promises to patients and stay compliant with regulations.
At Greenlight Guru, our QMS software ensures that all your documents and records live in the same system—breaking down silos, simplifying compliance, and keeping all your documentation organized, secure, and up-to-date.
Ready to see how Greenlight Guru can cut down the time you spend on managing all your documentation? Then get your free demo today!
Etienne Nichols is the Head of Industry Insights & Education at Greenlight Guru. As a Mechanical Engineer and Medical Device Guru, he specializes in simplifying complex ideas, teaching system integration, and connecting industry leaders. While hosting the Global Medical Device Podcast, Etienne has led over 200...
Related Posts
Let The Document Tell The Story: Document Management Best Practices
Engineering Change Order: Its Role in the Change Management Process
Leaning into Lean Documentation
Get your free resource
Checklist for Structuring your Technical Documentation










