How to Use an FMEA Template as Part of Your Medical Device Risk Management

Failure mode effects analysis (FMEA) is a popular tool for identifying the possible failures in the design of a product or process. FMEA is an industry-agnostic engineering method that makes it widely applicable to a variety of different situations, including medical device product development.
Though using FMEA alone will not satisfy the risk management requirements for MedTech companies laid out in ISO 14971:2019, an FMEA template can still be an effective tool to help you build a great device. Here’s how it works:
How does an FMEA template work?
A failure mode effects analysis consists of taking a component (or a part of a process), determining the potential ways it could fail, and then scoring those potential failures. There are three areas that must be scored, each on a scale of 1-10.
-
Severity (SEV) - This score is the severity of the consequences of a given failure mode. For example, a minor cosmetic defect would likely be scored very low. However, some effects—like the failure of an implantable device that is keeping someone alive—could be catastrophic in failure mode and would be given a high score.
-
Occurrence (OCC) - This represents the likelihood that the failure mode will occur. Using the same example from above, while the failure of the implantable device may lead to severe consequences, it may also be very unlikely; therefore, it would be given a low occurrence score. On the other hand, if the minor cosmetic issue occurs regularly, it would be given a much higher score.
-
Chance of detection (DET) - This score represents how well your current processes for detecting failure work. A high score here means you have little to no ability to detect a failure mode. If there is no process for detecting a failure mode, it should be given a score of 10.
Once you have all three scores, you then multiply them together to get what is known as your risk priority number (RPN). The higher the RPN, the higher the priority the potential failure should be for your company.
Your FMEA template should include this scoring, as well as elements like the potential effects of failure, potential causes of failure, your current risk controls, and potential mitigation activities. If you’re not sure where to start, you can download our free FMEA template that comes with everything you need to properly document your next FMEA.
Finally, keep in mind that the scoring of your RPN is a subjective process. So, when you’re conducting an FMEA, it’s important to assemble a cross-functional team composed of people with a good understanding of the design or process you are evaluating. Otherwise, your scoring may not accurately reflect the risk of a given failure mode.
Just remember that FMEA is not the same as risk management according to ISO 14971
FMEA is an excellent tool for identifying and mitigating the risk associated with the failure of specific components of your device, but you should not be using it as your sole risk management method.
That’s because there are risks inherent in medical devices that are beyond the scope of a potential failure of a device’s components. In addition to individual failure modes, there are also systemic risks that must be taken into account. To do so, you’ll need to use ISO 14971:2019, which lays out an approach to risk management based on the identification of hazards (potential source of harm) and hazardous situations (circumstance in which people, property, or the environment are exposed to one or more hazard(s)).
ISO 14971 also directs MedTech companies to estimate the severity of potential harms resulting from the hazards and hazardous situations, as well as the probability that those harms will occur. Severity of harm and probability of occurrence are what define risk.
Here’s a visual comparison between the two methods that should help clarify the difference in their scope:
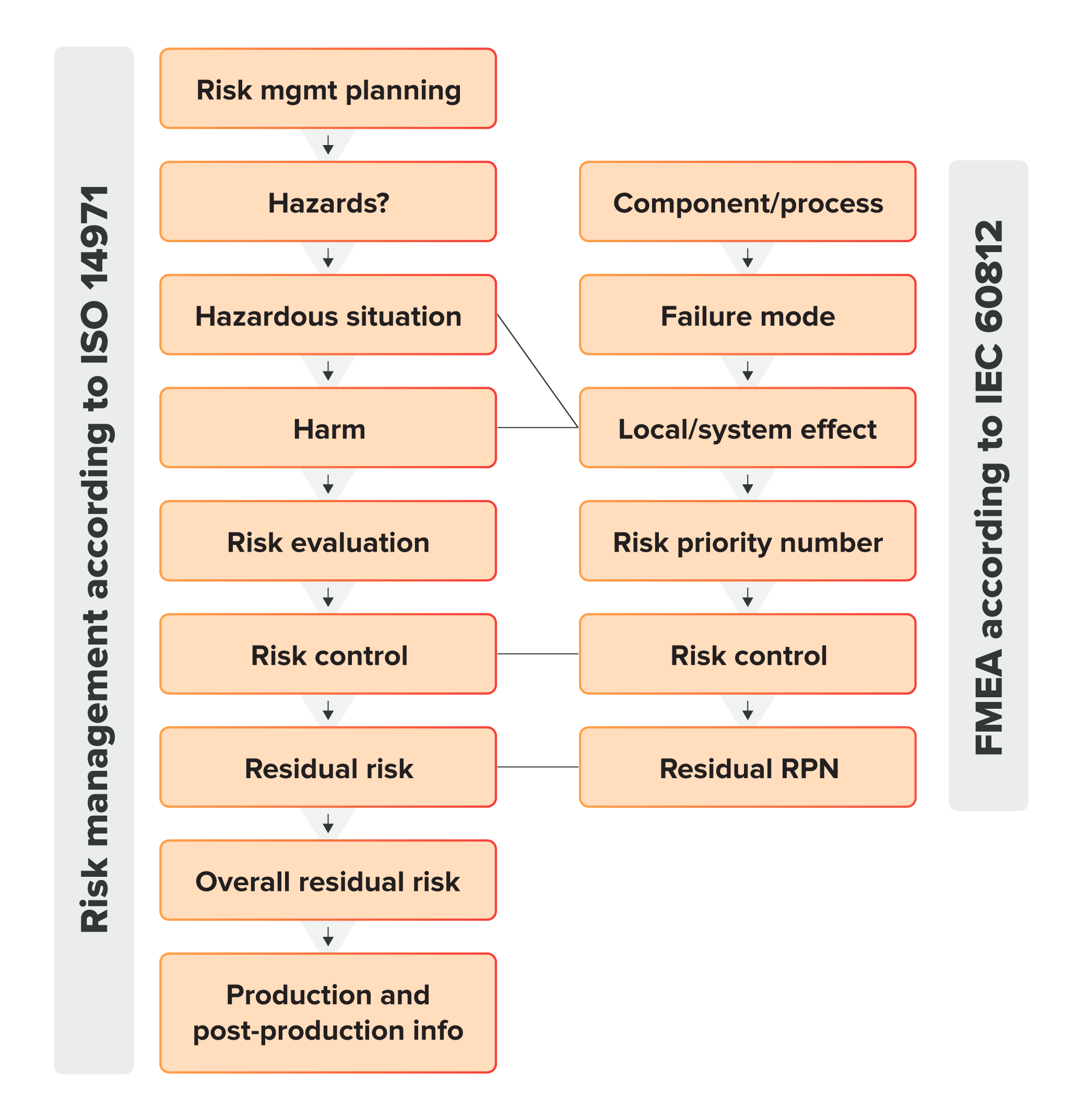
Now, keep in mind that while you do need to follow ISO 14971:2019 for your risk management activities, that doesn’t mean you can’t use FMEA. Forcing the analysis of every point of failure on your device is still a great way to build a device that rarely fails to work as it should, and FMEA can be used as a complementary process to ISO 14971:2019.
Integrate risk management throughout the entire medical device lifecycle with Greenlight Guru
Risk management is an essential part of bringing a safe, effective medical device to market and keeping it there long term. That’s why regulatory bodies around the world emphasize taking a risk-based approach throughout the lifecycle of a medical device.
At Greenlight Guru, we built our QMS software specifically for the MedTech industry, meaning our software is designed to comply with ISO 14971:2019 and the risk-based requirements of ISO 13485:2016. And because our Risk Management workspace is fully traceable with the rest of the QMS, you’ll always have a complete and accurate picture of risk throughout your device’s lifecycle.
So if you’re looking for a QMS solution that integrates risk into your entire quality ecosystem, then get your free demo of Greenlight Guru today!
Vernon Baker is a Mechanical Engineer and Senior Medical Device Guru with over a decade of experience in medical device quality, regulatory affairs, and product development. Formerly the Director of Engineering for a successful Class III (PMA) medical device manufacturer in the USA, Vernon brings deep expertise in...
Related Posts
Can dFMEA and ISO 14971 Co-Exist in Medical Device Risk Management?
The Role of dFMEA in Risk Management for Medical Devices
ISO 14971 Risk Management for Medical Devices: The Definitive Guide
Get your free resource
Resource Bundle for Risk Managers











