Clinical Trials in 2025: The Outlook for Medical Device Companies

In our 2025 Medical Device Industry Report, we surveyed more than 500 medical device professionals, asking questions about the industry and their own experiences working in MedTech right now.
One of the main emphases of the survey was on clinical affairs—specifically, how companies are approaching clinical activities and what they expect this year to hold in store. The answers, as always, were enlightening and sometimes surprising.
Given the importance of obtaining high-quality clinical data for both regulatory submissions and ongoing post-market surveillance, we wanted to dig deeper into the data from our report to offer an overview of what the clinical landscape looks like for medical device companies in 2025.
Here’s what we found.
BONUS RESOURCE: Click here to download the full 2025 Medical Device Industry Report for free!
Clinical trials are a top priority for medical device companies in 2025
Survey respondents from both pre-commercial and commercialized companies told us that clinical trials were one of their top objectives in 2025. However, respondents at pre-commercial companies were significantly more likely to say that pursuing clinical activities (including trials, clinical evaluations, and post-market clinical follow-up) was their top objective.
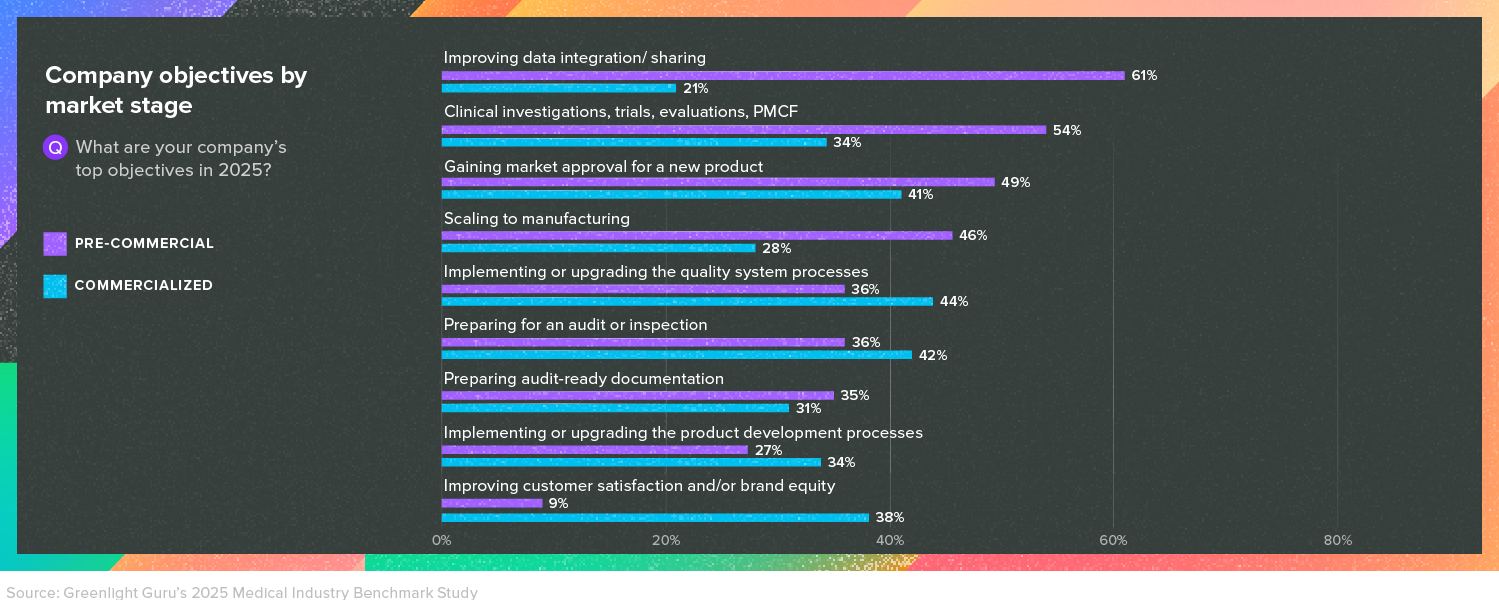
This isn’t surprising, as many pre-commercial companies will need clinical data to prove the safety and effectiveness of their devices during the regulatory submission process. Even devices that are not required by regulations to conduct a clinical trial may choose to do so in order to obtain clinical evidence that can boost market adoption.
Commercialized companies may also need to pursue clinical activities, especially in the EU, as the EU MDR has added more requirements around post-market surveillance, including the Post-Market Clinical Follow-up.
The top 3 challenges in medical device clinical trials
Despite the high value placed on clinical activities, just 47% of respondents with products on the market said they were equipped to successfully manage their clinical trials. When we asked what challenges these companies were facing, respondents gave us a variety of answers—but three stood out from the rest.
1. Funding for clinical trials
Funding was by far the most cited challenge in carrying out clinical trials.
.png)
The struggle for funding is of course related to the cost of clinical trials, which can quickly increase based on the number of participants and study sites and the cost of the devices used. But funding is also tied to the larger economic outlook, and our respondents stated that many of their companies were responding to economic uncertainty by pulling back on hiring and delaying new product development.
2. Difficulty with clinical data collection and management
The second most common problem our respondents cited with clinical trials was the collection and management of clinical data.
Another question in the report provides some clarity around why this is such a commonly cited struggle. When we asked what tools companies were using for clinical data collection, more than half of respondents told us they were using general-purpose tools or paper for clinical data collection.
.png)
And when it comes to obtaining accurate and compliant clinical data that can be used in a regulatory submission, these are the two most unreliable methods. Both paper and general-purpose tools (like Excel) open the door to human error and create enormous difficulties with compliance. It’s also extraordinarily difficult to run decentralized trials—which are quickly growing in popularity—with these tools.
At Greenlight Guru, we recommend every medical device company use electronic data capture (EDC) software to help ensure accurate data collection and compliant data management practices. Of course, the best EDC system for medical device companies is one built specifically for MedTech. That’s why Greenlight Guru Clinical comes aligned with ISO 14155:2020 and EU MDR and FDA regulations on clinical trials and data.
3. Recruitment challenges
The third most-cited challenge for medical device companies with clinical trials was patient recruitment. For many companies, this challenge can stem from the approach they take to recruitment, which is often an afterthought. Dr. Kelly Palmer made this point on a recent episode of the Global Medical Device Podcast. Sometimes the attitude toward building the recruitment protocol is, “If we build it they will come.”
But this attitude typically doesn’t lead to the desired recruitment numbers, which in turn extends study timelines well past the initial estimate.
Dr. Palmer found that going to the communities they wanted to recruit, meeting with people, and building relationships with them was key to hitting recruitment numbers. The more value the team could provide to the community, both monetary and otherwise, the more it made sense for people to sign up.
If you’re struggling with patient recruitment for a clinical trial, or you simply have an upcoming study, I’d really encourage you to listen to the whole episode—it’s full of great tips on how to overcome barriers to patient participation in your trials.
BONUS RESOURCE: Click here to download the full 2025 Medical Device Industry Report for free!
Solve your clinical struggles with an EDC system made for medical device companies
Some of the challenges that medical device companies are facing right now aren’t solvable. You can’t change macroeconomic conditions or regulations like EU MDR. But that doesn’t mean you’re powerless. It’s within your power to choose the tools and systems that will help you efficiently and cost-effectively build clinical studies, carry them out, and manage all your clinical data.
We built Greenlight Guru Clinical to be the only system that a medical device company will need for its clinical activities. Whether you’re creating a first-in-human study, running a trial for safety and effectiveness data, or sending out post-market surveys, you can do it all in Greenlight Guru Clinical.
Ready to see how a modern, MedTech-specific EDC system can keep your studies compliant and on-schedule? Then get your free demo of Greenlight Guru today!
Matt McFarlane is the Senior Content Writer at Greenlight Guru. He is an avid reader and writer, specializing in the medical device industry and its many regulations, standards, and guidance documents.
Related Posts
Ultimate Guide to Clinical Data Management in MedTech
2025 Medical Device Industry Report: Quality Challenges, Regulatory Complexity, and Economic Uncertainty
The Biggest Quality Challenges for Medical Device Companies in 2025
Get your free report
2025 Medical Device Industry Report










