AAMI TIR45: Closing the Gap Between Agile Software Development & Medical Device Regulations
In 2012, the Association for the Advancement of Medical Instrumentation (AAMI) released the technical report AAMI TIR45 - Guidance on the use of AGILE practices in the development of medical device software.
AAMI TIR45 filled a critical gap between Agile product development and the medical device world that was growing quickly at the time. Fast forward to today and TIR45 remains extremely relevant and useful—even more so with the growth of software as a medical device (SaMD).
So, let’s take a closer look to see how AAMI TIR45 bridges the gap between Agile software development and medical device regulations.
BONUS RESOURCE: Click here to download the free eBook of our Ultimate Guide to SaMD.
What is AAMI TIR45?
AAMI TIR45 is a guide for companies making medical device software that want to use Agile software development practices. This technical report offers practical guidance for applying Agile practices to traditional regulatory requirements in the medical device industry.
TIR45 was released by AAMI—an industry association made up of medical device manufacturers and the Food and Drug Administration (FDA)—in response to the growing use of Agile software development and perceptions that it was incompatible with medical device regulatory requirements.
Why was AAMI TIR45 necessary for medical device software development?
Agile is based on rapid release and iteration. And at first glance, this method appears to be at odds with FDA’s design controls. In fact, the famous image that FDA uses to illustrate design controls, looks as though it’s set up for waterfall project management.
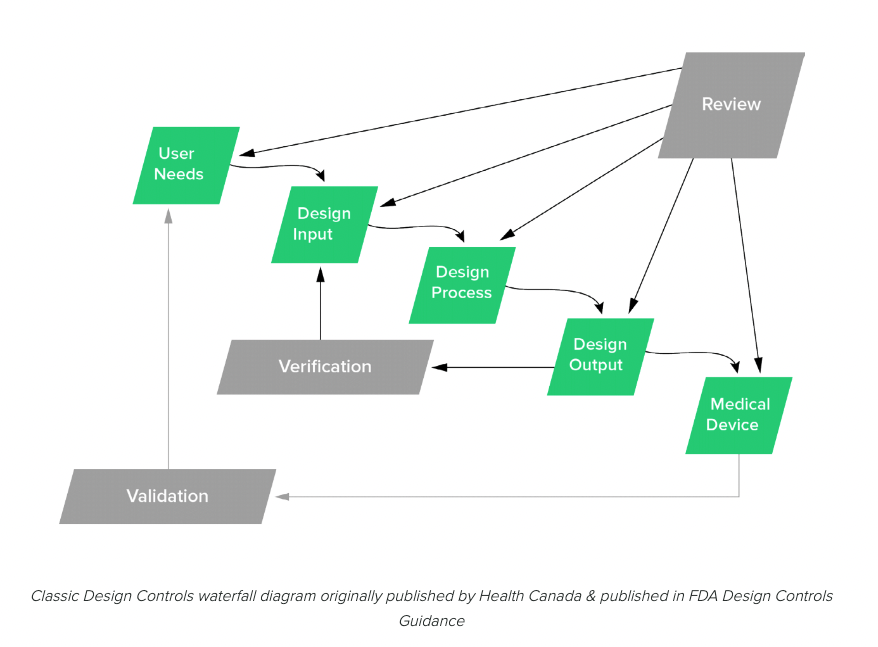
This diagram has led to a lot of angst for both software development teams and quality managers. The image strongly implies that FDA wants medical device manufacturers to use waterfall for product development—even for software.
Fortunately, this just isn’t true. The truth is, there is no conflict between Agile and regulatory requirements like design controls.
How does FDA view Agile software development in the context of AAMI TIR45?
While it’s true that regulations like the FDA’s Quality System Regulation were written before Agile became popular, FDA does not prescribe any one product development method.
Remember, FDA is part of AAMI, and TIR45 is an FDA consensus standard. FDA would not have recognized this technical report if they didn’t want people to use Agile.
However, using an Agile methodology doesn’t mean regulatory requirements related to design controls, documentation, or your quality management system can be avoided just because, “Agile doesn’t say we have to do that.”
In fact, AAMI TIR45’s initial recommendations for medical device manufacturers planning on using Agile for their software development make it clear that Agile practices must be used within the context of regulatory requirements:
-
Apply the values of Agile in a way that enhances a robust quality management system
-
Apply the practices of Agile within the context of an established quality management system
-
Set the correct expectations by defining the software development lifecycle model. Demonstrate how an incremental/evolutionary lifecycle satisfies regulatory requirements
-
Establish robust change management systems to manage changes and mitigate risks associated with rapid change.
Additionally, the authors of AAMI TIR45 also took into account a number of regulations and guidance documents while writing it, including:
-
IEC 62304
-
ISO 13485
-
ISO 14971
-
21 CFR Part 820
-
General Principles of Software Validation (FDA Guidance)
This is why AAMI TIR45 exists in the first place—to ensure that software developers in the medical device industry can use their preferred development methodology while staying compliant.
What are the benefits of Agile practices as laid out in AAMI TIR45?
Agile has long been used in software development because of its speed and flexibility compared to other product development methods. There are a wide range of Agile methodologies for executing the principles outlined in the original Manifesto for Agile Software Development, but they all focus on responsiveness to change and delivering software frequently.
AAMI TIR45 lists several advantages of Agile that the authors see as beneficial to medical device software development:
-
Continuous focus on safety, risk management, and delivering customer value through backlog prioritization, planning practices, and customer feedback
-
Continuous assessment of quality through continuous integration and testing
-
Continuous improvement of the software development process through retrospectives and team accountability
-
Continuous focus on “getting to done” and satisfying quality management stakeholders through the regular completion of activities and deliverables.
When done right, that focus on “continuous” assessment and improvement is a large part of what makes Agile such an effective method for developing software — especially in a highly regulated industry like MedTech.
BONUS RESOURCE: Click here to download the free PDF eBook of our Ultimate Guide to SaMD.
Choose a MedTech Lifecycle Excellence platform that’s as agile & intuitive as your software development practices
Agile changed the way we make software. It was a revolution that left behind outdated practices in favor of something newer and more effective.
At Greenlight Guru, we built our MedTech Lifecycle Excellence Platform according to that same ideal. Say goodbye to static spreadsheets, Word documents, or generic software solutions that take months to customize. Say hello to a purpose-built platform that does everything medical device companies need it to, from ideation to commercialization to post-market surveillance.
If you’re ready to use a platform built specifically for your company and the MedTech industry, then get your free demo of Greenlight Guru today.
Etienne Nichols is the Head of Industry Insights & Education at Greenlight Guru. As a Mechanical Engineer and Medical Device Guru, he specializes in simplifying complex ideas, teaching system integration, and connecting industry leaders. While hosting the Global Medical Device Podcast, Etienne has led over 200...
Related Posts
5 Dos and Don'ts when Choosing a QMS Solution for Your Medical Device Company
3 Life[cycle] Hacks for Integrating Risk Management throughout all Device Phases
Best Practices for an Effective Medical Device Design Transfer Process
Get your free eBook
Ultimate Guide to SaMD
%20Ultimate%20Guide%20to%20SaMD.png?width=250&name=(cover)%20Ultimate%20Guide%20to%20SaMD.png)

%20Ultimate%20Guide%20to%20SaMD.png?width=5100&name=(cover)%20Ultimate%20Guide%20to%20SaMD.png)







