2025 Medical Device Industry Report: Quality Challenges, Regulatory Complexity, and Economic Uncertainty

The medical device industry is growing. Data from KPMG predicts that global annual sales will rise by 5% per year to reach just under US$800 billion by 2030. New technology, new opportunities, and, as always, the promise of improving patient outcomes around the world are major drivers of growth within the industry.
But behind the projected growth lie significant challenges for individual medical device companies. Uncertain and changing economic conditions, new regulatory pressures, and challenges with managing quality are all quietly making themselves felt behind the scenes.
That’s why Greenlight Guru has released its 2025 Medical Device Industry Report—to pull back the curtain on the medical device industry and give readers a look into what their peers and competitors are saying about the state of the industry right now.
BONUS RESOURCE: Click here to download the full 2025 Medical Device Industry Report for free!
How we built this report
To create the report, we surveyed 536 quality, product development, clinical, and leadership professionals in the medical device industry over the course of October and November 2024 (check out the full report for a detailed demographic breakdown).
Our goal was to provide a holistic overview of the state of the medical device industry, along with deep dives into the quality and regulatory, product development, and clinical challenges these professionals are facing every day.
Rather than relying on market caps or the number of devices moved, we wanted to get into the details of what it’s like to be a medical device professional right now. Based on the responses from a wide range of professionals—from individual contributors up to company leadership—we were able to get detailed insights into how medical device companies are responding to the challenges and opportunities of 2025.
Key themes in the medical device industry in 2025
The results of our survey surfaced three key themes: quality challenges, regulatory complexity, and economic uncertainty. While the growth forecast for the medical device industry remains positive, individual companies are wrestling with these challenges as they make decisions about hiring, developing new products, and entering new markets.
Here’s a brief look at each of these themes:
1. Quality challenges
For both pre-commercial and commercialized companies, keeping their quality management system (QMS) audit-ready is typically a time-consuming task. However, the quality challenges only increase once a company places its first device on the market.
Greenlight Guru’s research shows that while pre-commercial companies say they spend 17 hours per month on reactive remediation activities, that number jumps to 52 hours for companies with products on the market.
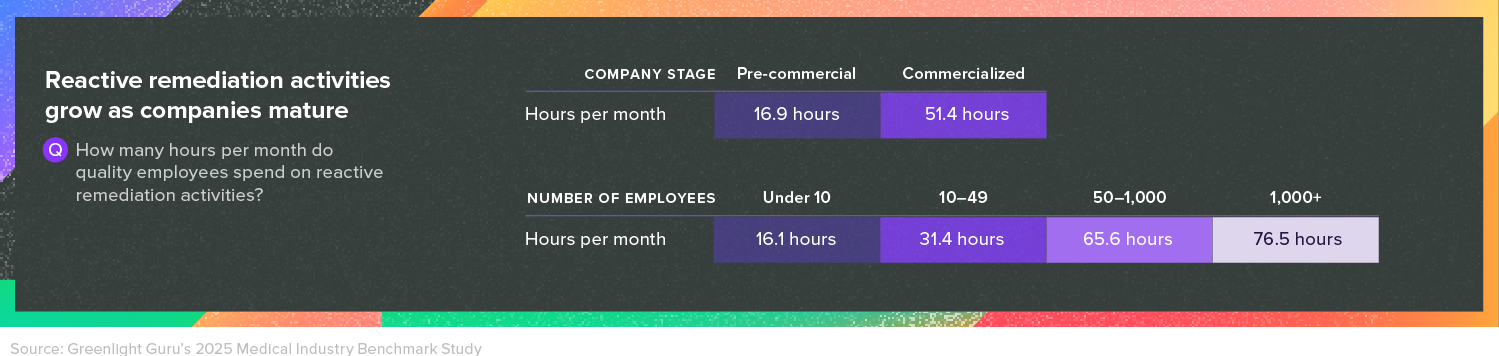
Part of the added time investment is that once a company places a device on the market, they must then carry out postmarket surveillance in accordance with the regulations in their market. And all of those postmarket surveillance activities must be integrated within the QMS. In the full report, you’ll learn what companies are doing to build excellent postmarket surveillance processes and bring down remediation times.
2. Regulatory complexity
Understanding and complying with medical device regulations (often in multiple markets) has never been easy, but with the continued challenges presented by EU MDR and the harmonization of ISO 13485:2016 and the QSR in the US, the degree of difficulty is as high as it has ever been.
Many respondents in both the US and the EU told us they were struggling with preparing for these regulations, though with striking differences in the reasons why.
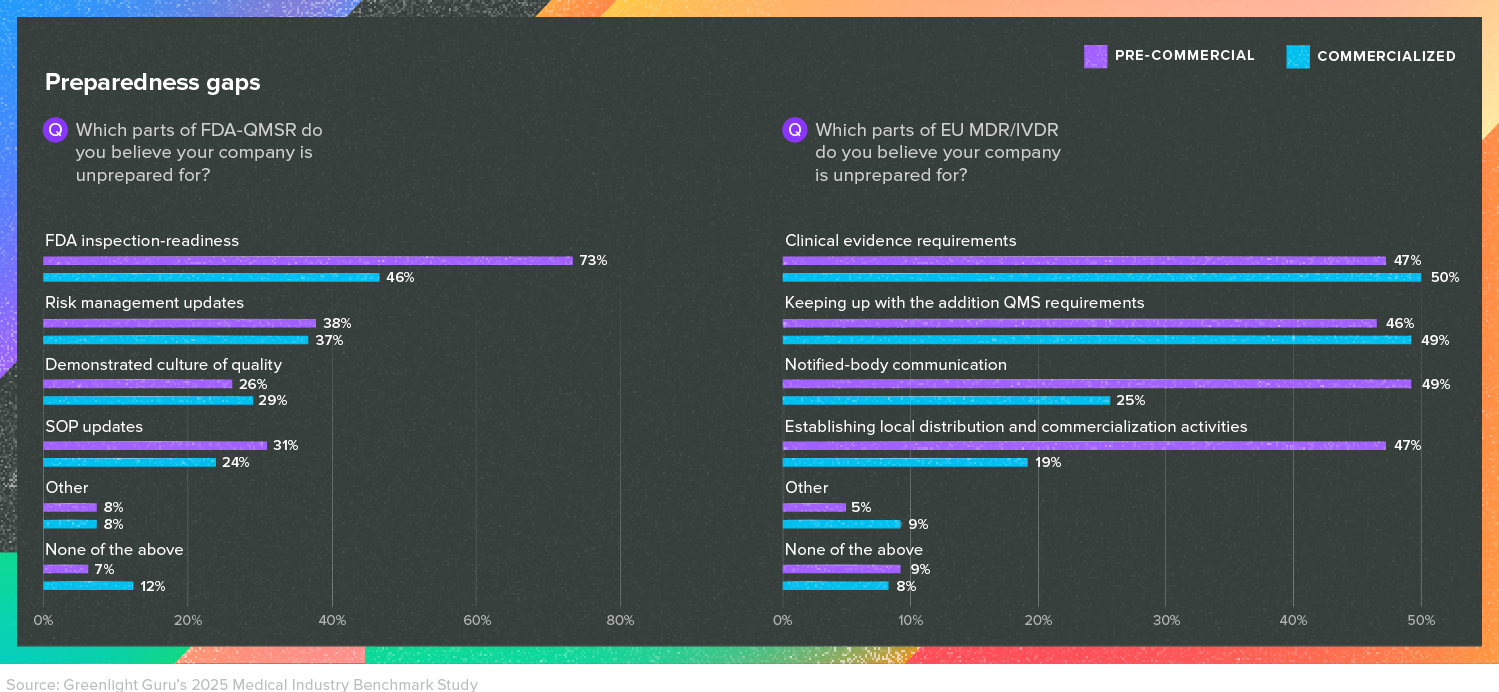
Keep in mind, results here were obtained prior to the current budgetary and staffing pressures applied to the Department of Health and Human Services in the US. While there isn’t a clear picture yet of how the FDA has been affected, it will only add to the level of uncertainty for companies that are already unsure of their preparedness for an FDA inspection.
However, the responses you’ll find in the report are not a result of recent actions—meaning regulatory complexity was already a challenge for medical device companies.
3. Economic uncertainty
Here, as well, recent developments were not taken into account in our survey. But even in late 2024, respondents were feeling uneasy about economic factors, with 46% of respondents from companies with more than 1,000 employees stating they had halted new hiring. Delays in new product development and technology investments, along with reduced headcounts, were also common answers.
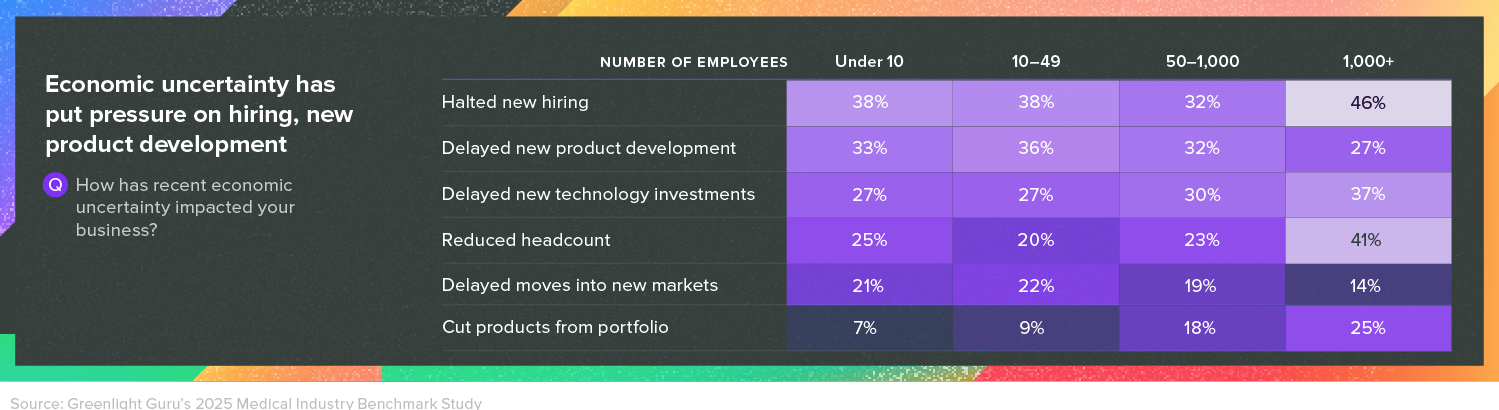
These numbers have huge implications, and it’s possible to see their effects elsewhere in the report, from submission timelines to technology purchases to entry into new markets—uncertainty tends to create drag.
BONUS RESOURCE: Click here to download the full 2025 Medical Device Industry Report for free!
Get the full report for a look inside the medical device industry
The statistics above are just a small piece of the full picture drawn in the report. To see more, you can download the entire 2025 Medical Device Industry Report for free.
Not only will you have a chance to see how your industry peers are approaching areas like clinical trials, new product development, new market entry, and much more. You’ll also get a look into some of the more common issues within organizations and how they affect both individuals and business as a whole. And finally, you’ll learn what medical device companies like yours can do to mitigate or avoid many of these issues altogether, and how strategic investments in both people and technology can change the way your company does business.
Matt McFarlane is the Senior Content Writer at Greenlight Guru. He is an avid reader and writer, specializing in the medical device industry and its many regulations, standards, and guidance documents.
Related Posts
Insights from an Ex-FDA Investigator: Compliance, Quality Systems, and MedTech Trends
FDA Guidance on Artificial Intelligence (AI) in Medical Devices
FDA Publishes Final Rule on QMSR
Get your free report
2025 Medical Device Industry Report











